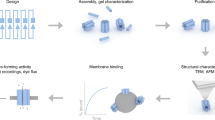
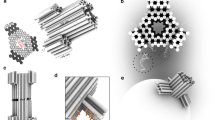
Abstract
The assembly of peptides into membrane-spanning nanopores might be promoted by scaffolds to pre-organize the structures. Such scaffolds could enable the construction of uniform pores of various sizes and pores with controlled permutations around a central axis. Here, we show that DNA nanostructures can serve as scaffolds to arrange peptides derived from the octameric polysaccharide transporter Wza to form uniform nanopores in planar lipid bilayers. Our ring-shaped DNA scaffold is assembled from short synthetic oligonucleotides that are connected to Wza peptides through flexible linkers. When scaffolded, the Wza peptides form conducting nanopores of which only octamers are stable and of uniform conductance. Removal of the DNA scaffold by cleavage of the linkers leads to a rapid loss of the nanopores from the lipid bilayer, which shows that the scaffold is essential for their stability. The DNA scaffold also adds functionality to the nanopores by enabling reversible and permanent binding of complementary tagged oligonucleotides near the nanopore entrance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Luckey, M. Membrane Structural Biology 2nd edn (Cambridge Univ. Press, Cambridge, 2014).
Zheng, J. & Trudeau M. C. (eds) Handbook of Ion Channels (CRC Press, Boca Raton, 2015).
Gilbert, R. J. C., Bayley, H. & Anderluh, G. Membrane pores: from structure and assembly, to medicine and technology. Phil. Trans. Roy. Soc. Lond. 372, 20160208 (2017).
Branton, D. et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 26, 1146–1153 (2008).
Ayub, M. & Bayley, H. Engineered transmembrane pores. Curr. Opin. Chem. Biol. 34, 117–126 (2016).
Koebnik, R., Locher, K. P. & van Gelder, P. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37, 239–253 (2000).
Tamm, L. K., Hong, H. & Liang, B. Folding and assembly of β-barrel membrane proteins. Biochim. Biophys. Acta 1666, 250–263 (2004).
Noinaj, N., Gumbart, J. C. & Buchanan, S. K. The beta-barrel assembly machinery in motion. Nat. Rev. Microbiol. 15, 197–204 (2017).
Dong, C. et al. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 444, 226–229 (2006).
Kong, L. et al. Single-molecule interrogation of a bacterial sugar transporter allows the discovery of an extracellular inhibitor. Nat. Chem. 5, 651–659 (2013).
Soskine, M. et al. An engineered ClyA nanopore detects folded target proteins by selective external association and pore entry. Nano Lett. 12, 4895–4900 (2012).
Mahendran, K. R. et al. A monodisperse transmembrane α-helical peptide barrel. Nat. Chem. 9, 411–419 (2017).
Mutter, M. & Vuilleumier, S. A chemical approach to protein design—template-assembled synthetic proteins (TASP). Angew. Chem. Int. Ed. 28, 535–554 (1989).
Futaki, S. Peptide ion channels: design and creation of function. Pept. Sci. 47, 75–81 (1998).
Bayley, H. & Jayasinghe, L. Functional engineered channels and pores (review). Mol. Membr. Biol. 21, 209–220 (2004).
Pinheiro, A. V., Han, D., Shih, W. M. & Yan, H. Challenges and opportunities for structural DNA nanotechnology. Nat. Nanotech. 6, 763–772 (2011).
Wilner, O. I., Shimron, S., Weizmann, Y., Wang, Z.-G. & Willner, I. Self-assembly of enzymes on DNA scaffolds: en route to biocatalytic cascades and the synthesis of metallic nanowires. Nano Lett. 9, 2040–2043 (2009).
Fu, J. et al. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm. Nat. Nanotech. 9, 531–536 (2014).
Zhang, Y., Tsitkov, S. & Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade. Nat. Commun. 7, 13982 (2016).
Raschle, T., Lin, C., Jungmann, R., Shih, W. M. & Wagner, G. Controlled co-reconstitution of multiple membrane proteins in lipid bilayer nanodiscs using DNA as a scaffold. ACS Chem. Biol. 10, 2448–2454 (2015).
Lee, H. et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotech. 7, 389–393 (2012).
Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 335, 831–834 (2012).
Langecker, M. et al. Synthetic lipid membrane channels formed by designed DNA nanostructures. Science 338, 932–936 (2012).
Burns, J. R., Stulz, E. & Howorka, S. Self-assembled DNA nanopores that span lipid bilayers. Nano Lett. 13, 2351–2356 (2013).
Karshikoff, A., Nilsson, L. & Ladenstein, R. Rigidity versus flexibility: the dilemma of understanding protein thermal stability. FEBS J. 282, 3899–3917 (2015).
von Krbek, L. K. S. et al. The delicate balance of preorganisation and adaptability in multiply Bonded host–guest complexes. Chem. Eur. J. 23, 2877–2883 (2017).
Jolliffe, K. A. Backbone-modified cyclic peptides: new scaffolds for supramolecular chemistry. Supramol. Chem. 17, 81–86 (2005).
Song, C. et al. Crystal structure and functional mechanism of a human antimicrobial membrane channel. Proc. Natl Acad. Sci. USA 110, 4586–4591 (2013).
Bowie, J. U. Solving the membrane protein folding problem. Nature 438, 581–589 (2005).
Krasilnikov, O. V. et al. Electrophysiological evidence for heptameric stoichiometry of ion channels formed by Staphylococcus aureus alpha-toxin in planar lipid bilayers. Mol. Microbiol. 37, 1372–1378 (2000).
Salay, L. C., Procopio, J., Oliveira, E., Nakaie, C. R. & Schreier, S. Ion channel-like activity of the antimicrobial peptide tritrpticin in planar lipid bilayers. FEBS Lett. 565, 171–175 (2004).
Paulmann, M. et al. Structure–activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J. Biol. Chem. 287, 8434–8443 (2012).
Howorka, S. & Bayley, H. Probing distance and electrical potential within a protein pore with tethered DNA. Biophys. J. 83, 3202–3210 (2002).
Rothemund, P. W. K. Folding DNA to create nanoscale shapes and patterns. Nature 440, 297–302 (2006).
Soskine, M., Biesemans, A., De Maeyer, M. & Maglia, G. Tuning the size and properties of ClyA nanopores assisted by directed evolution. J. Am. Chem. Soc. 135, 13456–13463 (2013).
Nicol, F., Nir, S. & Szoka, F. C. Orientation of the pore-forming peptide GALA in POPC vesicles determined by a BODIPY-avidin/biotin binding assay. Biophys. J. 76, 2121–2141 (1999).
Li, W., Nicol, F. & Szoka, F. C. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 56, 967–985 (2004).
Rausch, J. M., Marks, J. R. & Wimley, W. C. Rational combinatorial design of pore-forming β-sheet peptides. Proc. Natl Acad. Sci. USA 102, 10511–10515 (2005).
Krauson, A. J., He, J., Wimley, A. W., Hoffmann, A. R. & Wimley, W. C. Synthetic molecular evolution of pore-forming peptides by iterative combinatorial library screening. ACS Chem. Biol. 8, 823–831 (2013).
Gupta, K., Singh, S. & van Hoek, L. M. Short, synthetic cationic peptides have antibacterial activity against Mycobacterium smegmatis by forming pores in membrane and synergizing with antibiotics. Antibiotics 4, 358–378 (2015).
Thomson, A. R. et al. Computational design of water-soluble α-helical barrels. Science 346, 485 (2014).
Yusko, E. C. et al. Real-time shape approximation and fingerprinting of single proteins using a nanopore. Nat. Nanotech. 12, 360–367 (2017).
Huang, G., Willems, K., Soskine, M., Wloka, C. & Maglia, G. Electro-osmotic capture and ionic discrimination of peptide and protein biomarkers with FraC nanopores. Nat. Commun. 8, 935 (2017).
Soskine, M., Biesemans, A. & Maglia, G. Single-molecule analyte recognition with ClyA nanopores equipped with internal protein adaptors. J. Am. Chem. Soc. 137, 5793–5797 (2015).
Wanunu, M. et al. Discrimination of methylcytosine from hydroxymethylcytosine in DNA molecules. J. Am. Chem. Soc. 133, 486–492 (2011).
Taylor, A. I. et al. Nanostructures from synthetic genetic polymers. ChemBioChem 17, 1107–1110 (2016).
Hammerstein, A. F., Jayasinghe, L. & Bayley, H. Subunit dimers of α-hemolysin expand the engineering toolbox for protein nanopores. J. Biol. Chem. 286, 14324–14334 (2011).
Lee, J. & et al. Semisynthetic nanoreactor for reversible single-molecule covalent chemistry. ACS Nano 10, 8843–8850 (2016).
Williams, B. A. R. & Chaput, J. C. in S. L. Beaucage (ed.) Current Protocols in Nucleic Acid Chemistry (Wiley, Hoboken, 2010).
Gutsmann, T., Heimburg, T., Keyser, U., Mahendran, K. R. & Winterhalter, M. Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat. Protoc. 10, 188–198 (2015).
Acknowledgements
E.S. acknowledges support from the European Union's Horizon 2020 Programme under grant 655660 (Hybripore). The work was supported by the BBSRC and a European Research Council Advanced Grant. The authors thank O. Wilner and K. R. Mahendran for their helpful discussions, E. Johnson for help with the TEM measurements and I. Liko and C. V. Robinson for their assistance in mass spectrometry.
Author information
Authors and Affiliations
Contributions
E.S. and H.B. conceived and designed the experiments and wrote the paper. E.S. performed the experiments and simulations and analysed data. S.T. performed photobleaching experiments and analysed data. All the authors discussed and commented on the manuscript.
Corresponding authors
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Data 1–4 and Supplementary References
Supplementary Video 1
OxDNA2 coarse-grained MD simulation of the DNA scaffold without external force applied to the arms. Duration, 0.152 ms; frame rate, 16.4 µs–1.
Supplementary Video 2
OxDNA2 coarse-grained MD simulation of the DNA scaffold with an external force applied to all terminal 3ʹ and 5ʹ nucleotides in the direction perpendicular to the plane of the ring leading to a down-pointing orientation of all arms within 5 µs. Duration, 30 µs; frame rate, 11.6 µs–1.
Rights and permissions
About this article
Cite this article
Spruijt, E., Tusk, S.E. & Bayley, H. DNA scaffolds support stable and uniform peptide nanopores. Nature Nanotech 13, 739–745 (2018). https://doi.org/10.1038/s41565-018-0139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0139-6
This article is cited by
-
Highly shape- and size-tunable membrane nanopores made with DNA
Nature Nanotechnology (2022)
-
Nanopore-based technologies beyond DNA sequencing
Nature Nanotechnology (2022)
-
De novo design of transmembrane nanopores
Science China Chemistry (2022)
-
Bottom-up fabrication of a proteasome–nanopore that unravels and processes single proteins
Nature Chemistry (2021)
-
Constructing ion channels from water-soluble α-helical barrels
Nature Chemistry (2021)