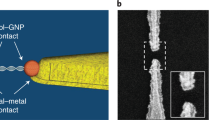
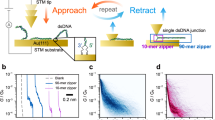
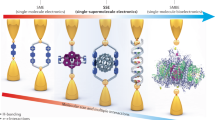
Abstract
Self-assembling circuit elements, such as current splitters or combiners at the molecular scale, require the design of building blocks with three or more terminals. A promising material for such building blocks is DNA, wherein multiple strands can self-assemble into multi-ended junctions, and nucleobase stacks can transport charge over long distances. However, nucleobase stacking is often disrupted at junction points, hindering electric charge transport between the two terminals of the junction. Here, we show that a guanine-quadruplex (G4) motif can be used as a connector element for a multi-ended DNA junction. By attaching specific terminal groups to the motif, we demonstrate that charges can enter the structure from one terminal at one end of a three-way G4 motif, and can exit from one of two terminals at the other end with minimal carrier transport attenuation. Moreover, we study four-way G4 junction structures by performing theoretical calculations to assist in the design and optimization of these connectors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Seeman, N. C. DNA in a material world. Nature 421, 427–431 (2003).
Seeman, N. C. Nanomaterials based on DNA. Annu. Rev. Biochem. 79, 65–87 (2010).
Genereux, J. C. & Barton, J. K. Mechanisms for DNA charge transport. Chem. Rev. 110, 1642–1662 (2010).
Huang, Y. C., Cheng, A. K. H., Yu, H.-Z. & Sen, D. Charge conduction properties of a parallel-stranded DNA G-quadruplex: implications for chromosomal oxidative damage. Biochemistry 48, 6794–6804 (2009).
Livshits, G. I. et al. Long-range charge transport in single G-quadruplex DNA molecules. Nat. Nanotech. 9, 1040–1046 (2014).
Xiang, L. et al. Intermediate tunnelling–hopping regime in DNA charge transport. Nat. Chem. 7, 221–226 (2015).
Huang, Y. C. & Sen, D. A twisting electronic nanoswitch made of DNA. Angew. Chem. Int. Ed. 53, 14055–14059 (2014).
Odom, D. T., Dill, E. A. & Barton, J. K. Robust charge transport in DNA double crossover assemblies. Chem. & Biol. 7, 475–481 (2000).
Odom, D. T., Dill, E. A. & Barton, J. K. Charge transport through DNA four-way junctions. Nucl Acids Res. 29, 2026–2033 (2001).
Young, R. M. et al. Charge transport across DNA-based three-way junctions. J. Am. Chem. Soc. 137, 5113–5122 (2015).
Liu, B., Leontis, N. B. & Seeman, N. C. Bulged 3-arm DNA branched junctions as components for nanoconstruction. Nanobiology 3, 177–188 (1994).
Zhang, Y. et al. Conformationally gated charge transfer in DNA three-way junctions. J. Phys. Chem. Lett. 6, 2434–2438 (2015).
Gellert, M., Lipsett, M. N. & Davies, D. R. Helix formation by guanylic acid. Proc. Natl Acad. Sci. USA 48, 2013–2018 (1962).
Venczel, E. A. & Sen, D. Synapsable DNA. J. Mol. Biol. 257, 219–224 (1996).
Venkatramani, R., Wierzbinski, E., Waldeck, D. H. & Beratan, D. N. Breaking the simple proportionality between molecular conductances and charge transfer rates. Farad. Discuss. 174, 57–78 (2014).
Xu, B. & Tao, N. J. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 301, 1221–1223 (2003).
Fahlman, R. P. & Sen, D. Cation-regulated self-association of ‘synapsable’ DNA duplexes. J. Mol. Biol. 280, 237–244 (1998).
Woiczikowski, P. B., Kubař, T., Gutiérrez, R., Cuniberti, G. & Elstner, M. Structural stability versus conformational sampling in biomolecular systems: Why is the charge transfer efficiency in G4-DNA better than in double-stranded DNA? J. Chem. Phys. 133, 035103 (2010).
Giese, B. Long-distance charge transport in DNA: the hopping mechanism. Acc. Chem. Res. 33, 631–636 (2000).
Jortner, J., Bixon, M., Langenbacher, T. & Michel-Beyerle, M. E. Charge transfer and transport in DNA. Proc. Natl Acad. Sci. USA 95, 12759–12765 (1998).
Sugiyama, H. & Saito, I. Theoretical studies of GG-specific photocleavage of DNA via electron transfer: significant lowering of ionization potential and 5′-localization of HOMO of stacked GG bases in B-Form DNA. J. Am. Chem. Soc. 118, 7063–7068 (1996).
Berlin, Y. A., Burin, A. L. & Ratner, M. A. On the long-range charge transfer in DNA. J. Phys. Chem. A 104, 443–445 (2000).
Hush, N. S. & Cheung, A. S. Ionization potentials and donor properties of nucleic acid bases and related compounds. Chem. Phys. Lett. 34, 11–13 (1975).
Lewis, F. D., Letsinger, R. L. & Wasielewski, M. R. Dynamics of photoinduced charge transfer and hole transport in synthetic DNA hairpins. Acc. Chem. Res. 34, 159–170 (2000).
Giese, B., Amaudrut, J., Kohler, A.-K., Spormann, M. & Wessely, S. Direct observation of hole transfer through DNA by hopping between adenine bases and by tunneling. Nature 412, 318–320 (2001).
Kawai, K. & Majima, T. Hole transfer kinetics of DNA. Acc. Chem. Res. 46, 2616–2625 (2013).
Liu, C. et al. Engineering nanometre-scale coherence in soft matter. Nat. Chem. 8, 941–945 (2016).
Lech, C. J., Phan, A. T., Michel-Beyerle, M.-E. & Voityuk, A. A. Electron-hole transfer in G-quadruplexes with different tetrad stacking geometries: a combined QM and MD Study. J. Phys. Chem. B 117, 9851–9856 (2013).
Lech, C. J., Phan, A. T., Michel-Beyerle, M.-E. & Voityuk, A. A. Influence of base stacking geometry on the nature of excited states in G-quadruplexes: a time-dependent DFT Study. J. Phys. Chem. B 119, 3697–3705 (2015).
Changenet-Barret, P., Hua, Y. & Markovitsi, D. Electronic excitations in guanine quadruplexes. Top. Curr. Chem. 356, 183–201 (2014).
Voityuk, A. A., Rösch, N., Bixon, M. & Jortner, J. Electronic coupling for charge transfer and transport in DNA. J. Phys. Chem. B 104, 9740–9745 (2000).
Nitzan, A. Chemical Dynamics in Condensed Phases: Relaxation, Transfer, and Reactions in Condensed Molecular Systems (Oxford University Press, Oxford, 2006).
Blumberger, J. Recent advances in the theory and molecular simulation of biological electron transfer reactions. Chem. Rev. 115, 11191–11238 (2015).
Zhang, Y. et al. DNA charge transport: moving beyond 1D. Surf. Sci. 652, 33–38 (2016).
Balaeff, A., Craig, S. L. & Beratan, D. N. B-DNA to Zip-DNA: Simulating a DNA transition to a novel structure with enhanced charge-transport characteristics. J. Phys. Chem. A 115, 9377–9391 (2011).
Chen, F., Li, X., Hihath, J., Huang, Z. & Tao, N. Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. J. Am. Chem. Soc. 128, 15874–15881 (2006).
Caruthers, M. H. Gene synthesis machines: DNA chemistry and its uses. Science 230, 281–285 (1985).
Xu, B., Zhang, P., Li, X. & Tao, N. Direct conductance measurement of single DNA molecules in aqueous solution. Nano Lett. 4, 1105–1108 (2004).
Guo, S., Hihath, J., Díez-Pérez, I. & Tao, N. Measurement and statistical analysis of single-molecule current–voltage characteristics, transition voltage spectroscopy, and tunneling barrier height. J. Am. Chem. Soc. 133, 19189–19197 (2011).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Sen, S. & Nilsson, L. MD simulations of homomorphous PNA, DNA, and RNA single strands: characterization and comparison of conformations and dynamics. J. Am. Chem. Soc. 123, 7414–7422 (2001).
Scholes, G. D. Long-range resonance energy transfer in molecular systems. Annu. Rev. Phy. Chem. 54, 57–87 (2003).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian, Inc., Wallingford, CT, 2009).
Acknowledgements
We thank the Office of Naval Research (N00014-11-1-0729) for support.
Author information
Authors and Affiliations
Contributions
R.S. and N.C.S. designed and synthesized the DNA molecules. L.X., Y.L. and N.T. designed and conducted the conductance measurements experiments. C.L., A.B., Y.Z., P.Z. and D.N.B. conducted and analysed the simulations. The three teams collaborated intensively in formulating the key molecular designs, analysing the data and writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–11, Supplementary Tables 1–2, Supplementary Notes 1–3.
Rights and permissions
About this article
Cite this article
Sha, R., Xiang, L., Liu, C. et al. Charge splitters and charge transport junctions based on guanine quadruplexes. Nature Nanotech 13, 316–321 (2018). https://doi.org/10.1038/s41565-018-0070-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0070-x
This article is cited by
-
Understanding self-assembly at molecular level enables controlled design of DNA G-wires of different properties
Nature Communications (2022)
-
From molecular to supramolecular electronics
Nature Reviews Materials (2021)
-
Single-molecule junction spontaneously restored by DNA zipper
Nature Communications (2021)
-
Towards single-molecule optoelectronic devices
Science China Chemistry (2018)