Abstract
Measles remains a major threat to human health despite widespread vaccination. While we know that maternal antibodies can impair vaccine-induced immunity, the relative contributions of pre-existing immunity levels, maternal and infant characteristics on vaccine responses remain unclear, hampering evidence-based vaccination policy development. Here we combine serological data from 1,505 individuals (aged 0–12 years) in a mother–infant cohort and in a child cohort with empirical models to reconstruct antibody trajectories from birth. We show that while highly heterogeneous across a population, measles antibody evolution is strongly predictive from birth at the individual level, including following vaccination. Further, we find that caesarean section births were linked with 2.56 (95% confidence interval: 1.06–6.37) increased odds of primary vaccine failure, highlighting the long-term immunological consequences of birth route. Finally, we use our new understanding of antibody evolution to critically assess the population-level consequences of different vaccination schedules, the results of which will allow country-level evaluations of vaccine policy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Because of privacy and ethical reasons, individual epidemiological and serological data cannot be made public, but they are available from the corresponding author (H.Y.) on reasonable request. The time frame for a response to requests is 1–2 weeks. We have provided the synthetic dataset, and aggregated de-identified data generated in this study. These datasets have been deposited in Zenodo at https://doi.org/10.5281/zenodo.11018560 (ref. 35). Source data are provided with this paper.
Code Availability
The codes to reproduce the main results and Extended data in this study have been deposited in Zenodo at https://doi.org/10.5281/zenodo.11018560 (ref. 35).
References
Minta, A. A. et al. Progress toward regional measles elimination—worldwide, 2000–2021. MMWR Morb. Mortal. Wkly Rep. 71, 1489–1495 (2022).
Ma, C. et al. Progress toward measles elimination—China, January 2013–June 2019. MMWR Morb. Mortal. Wkly Rep. 68, 1112–1116 (2019).
Patel, M. et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb. Mortal. Wkly Rep. 68, 402–404 (2019).
Wiedermann, U., Garner-Spitzer, E. & Wagner, A. Primary vaccine failure to routine vaccines: why and what to do? Hum. Vaccin. Immunother. 12, 239–243 (2016).
Failure to vaccinate and vaccine failure. Nat. Microbiol. 4, 725 (2019).
Guerra, F. M. et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect. Dis. 17, e420–e428 (2017).
Nic Lochlainn, L. M. et al. Effect of measles vaccination in infants younger than 9 months on the immune response to subsequent measles vaccine doses: a systematic review and meta-analysis. Lancet Infect. Dis. 19, 1246–1254 (2019).
Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 32, e00084–18 (2019).
Cáceres, V. M., Strebel, P. M. & Sutter, R. W. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin. Infect. Dis. 31, 110–119 (2000).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Carazo, S., Billard, M.-N., Boutin, A. & De Serres, G. Effect of age at vaccination on the measles vaccine effectiveness and immunogenicity: systematic review and meta-analysis. BMC Infect. Dis. 20, 251 (2020).
Leuridan, E. et al. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ 340, c1626 (2010).
Wang, Q. et al. Long-term measles antibody profiles following different vaccine schedules in China, a longitudinal study. Nat. Commun. 14, 1746 (2023).
Wei, X. et al. The transfer and decay of maternal antibodies against enterovirus A71, and dynamics of antibodies due to later natural infections in Chinese infants: a longitudinal, paired mother-neonate cohort study. Lancet Infect. Dis. 21, 418–426 (2021).
Steinhoff, M. C. et al. Influenza immunization in pregnancy–antibody responses in mothers and infants. N. Engl. J. Med. 362, 1644–1646 (2010).
Perret, C. et al. Dengue infection during pregnancy and transplacental antibody transfer in Thai mothers. J. Infect. 51, 287–293 (2005).
Metcalf, C. J. E. et al. Comparing the age and sex trajectories of SARS-CoV-2 morbidity and mortality with other respiratory pathogens. R. Soc. Open Sci. 9, 211498 (2022).
Furman, D. et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. USA 111, 869–874 (2014).
van Panhuis, W. G. et al. Decay and persistence of maternal dengue antibodies among infants in Bangkok. Am. J. Trop. Med. Hyg. 85, 355–362 (2011).
Waaijenborg, S. et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J. Infect. Dis. 208, 10–16 (2013).
Teunis, P. F. M., van Eijkeren, J. C. H., de Graaf, W. F., Marinović, A. B. & Kretzschmar, M. E. E. Linking the seroresponse to infection to within-host heterogeneity in antibody production. Epidemics 16, 33–39 (2016).
Li, H.-T., Hellerstein, S., Zhou, Y.-B., Liu, J.-M. & Blustein, J. Trends in Cesarean delivery rates in China, 2008–2018. JAMA 323, 89–91 (2020).
de Koff, E. M. et al. Mode of delivery modulates the intestinal microbiota and impacts the response to vaccination. Nat. Commun. 13, 6638 (2022).
Liu, H. et al. Vaginal delivery and breastfeeding benefit infant immune response to hepatitis B vaccine: a prospective cohort study. J. Clin. Transl. Hepatol. 11, 899–907 (2023).
Li, H. et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 584, 274–278 (2020).
Strömbeck, A. et al. Earlier infantile immune maturation is related to higher DTP vaccine responses in children. Clin. Transl. Immunol. 5, e65 (2016).
Rosenthal, S. R. & Clements, C. J. Two-dose measles vaccination schedules. Bull. World Health Organ. 71, 421–428 (1993).
Chen, R. T. et al. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162, 1036–1042 (1990).
Launay, O. et al. Safety and immunogenicity of a measles-vectored SARS-CoV-2 vaccine candidate, V591 / TMV-083, in healthy adults: results of a randomized, placebo-controlled Phase I study. EBioMedicine 75, 103810 (2022).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Amanna, I. J. & Slifka, M. K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 411, 206–215 (2011).
Yang, J. et al. Seroepidemiology of enterovirus A71 infection in prospective cohort studies of children in southern China, 2013–2018. Nat. Commun. 13, 7280 (2022).
He, Y. et al. Assessing vaccination coverage, timeliness, and its temporal variations among children in a rural area in China. Hum. Vaccin. Immunother. 17, 592–600 (2021).
Argüelles, M. H. et al. Measles virus-specific antibody levels in individuals in Argentina who received a one-dose vaccine. J. Clin. Microbiol. 44, 2733–2738 (2006).
Yu Group. Analysis data and code for the measles_Ab_dynamics. Zenodo https://doi.org/10.5281/zenodo.11018560 (2024).
Acknowledgements
We thank all contributors and study participants for their participation and trust; Fudan University for providing the computational resources to perform this study and data management support. H.Y. acknowledges financial support from the Key Program of the National Natural Science Foundation of China (82130093). W.W. acknowledges financial support from the Young Scientists Fund of the National Natural Science Foundation of China (82304205), the National Postdoctoral Program for Innovative Talent (BX2021072) and the China Postdoctoral Science Foundation (2022M720753). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We also acknowledge B. T. Grenfell and C. J. E. Metcalf of Princeton University, and A. T. Huang of the University of Cambridge for helpful comments on the paper.
Author information
Authors and Affiliations
Contributions
H.S. and H.Y. conceived the study. Q.W. and S.Z. performed the laboratory tests. W.W. collated data and performed the analysis. M.O. reviewed the code. W.W., M.O. and H.S. wrote the first draft of the paper. W.W., M.O. H.S. and H.Y. discussed the results and contributed to revisions of the paper.
Corresponding authors
Ethics declarations
Competing interests
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, Shanghai Roche Pharmaceutical Company, and SINOVAC Biotech Ltd. None of these research fundings is related to measles. The other authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
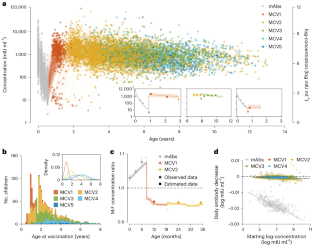
Extended Data Fig. 1 Age- and sex-specific concentration distributions.
(a) Number of observations and (b) measured concentrations (mIU/ml) by age and sex. In (b), the points with error bars represent the measured mean concentrations and the 95% confidence intervals for 555 adult women participants, as well as for 554, 328, 774, 1004, 820 and 478 participants aged 0 months (301 boys and 253 girls), 6 months (175 boys and 153 girls), 1–2 years (390 boys and 384 girls), 3–4 years (509 boys and 495 girls), 5–6 years (408 boys and 412 girls) and 7–11 years (235 boys and 243 girls), respectively. The abbreviation ‘M’ denotes the mothers in the mother-infant cohort.
Extended Data Fig. 2 Comparison of measured log-concentrations and log-concentration estimates from Model A.
(a–g) The consistency between measured and model estimated log-concentrations (log mIU/ml) among 350, 350, 224, 276, 350, 235 and 226 participants who were followed at 0, 2, 4, 6, 12, 24 and 36 months of age. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model A, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions (n = 3,000).
Extended Data Fig. 3 Differences in vaccine responses between MCV1 responders and non-responders.
(a) Probability density distribution of MCV1-induced peak log-concentration increase (log mIU/ml); (b) pre-MCV1, (c) MCV1-induced and (d) post-MCV1 log-concentrations (log mIU/ml) in MCV1 responders (n = 324) and non-responders (n = 26). Note that the dashed line in (a) is used to differentiate the peak log-concentration rise from MCV1 in MCV1 non-responders (left curve) and responders (right curve); in (b–d), each box plot shows minimum, first quartile, median, third quartile, and maximum values, while each point represents individual prediction.
Extended Data Fig. 4 The blunting of primary vaccine response due to maternal antibodies.
Note that the solid lines and shaded areas show the mean of posterior median estimated concentrations (mIU/ml) and the 95% credible intervals in each quartile group.
Extended Data Fig. 5 Comparison of measured and estimated log-concentrations before and after MCV1 vaccination.
(a–g) The consistency between measured and estimated log-concentrations (log mIU/ml) among 350 cohort women and 350, 350, 224, 276, 350, and 9 child participants who were followed at 0, 2, 4, 6, 12 and 24 months of age. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model C, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions (n = 3,000).
Extended Data Fig. 6 Comparison of measured log-concentrations and log-concentration estimates from model B in the mother-infant cohort.
(a–g) The consistency between measured and estimated log-concentrations (log mIU/ml) from 350, 350, 224, 276, 350, 235 and 226 participants aged 0, 2, 4, 6, 12, 24 and 36 months. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model B, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions (n = 3,000).
Extended Data Fig. 7 Comparison of measured log-concentrations and log-concentration estimates from model B in the child cohort.
(a–g) The consistency between measured and estimated log-concentrations (log mIU/ml) from 1148, 325, 1033, 303, 1031, 306 and 1061 participants at baseline and six subsequent follow-up visits. The black dotted line represents 100% consistency between measured and estimated log-concentrations, while the green line is the loess line through the data points. The grey vertical bar in each data point (mean) shows the 95% credible interval for individual antibody concentration from the model B, which is calculated from the 2.5% and 97.5% percentiles of the posterior distributions (n = 3,000).
Extended Data Fig. 8 The validation of the expanded model framework.
Note that the filled points with error bars show the measured mean concentrations (mIU/ml) and the 95% confidence intervals for 350, 350, 268, 298, 102, 237, 78, 262, 41, 91, 22 participants who were followed at 0 (350 in the infant cohort), 2–5 (350 in the infant cohort), 6–11 (268 in the infant cohort), 12–17 (86 in the infant cohort, 212 in the child cohort), 18–23 (35 in the infant cohort, 67 in the child cohort), 24–29 (85 in the infant cohort, 152 in the child cohort), 30–35 (37 in the infant cohort, 41 in the child cohort), 36–41 (90 in the infant cohort, 172 in the child cohort), 42–47 (41 in the child cohort), 48–53 (91 in the child cohort), and 54–60 (22 in the child cohort) months. The open points with error bars show the predicted mean concentrations and the 95% credible intervals (n = 10,000). The points in grey, red and yellow areas indicate the mean estimates of maternal, post-MCV1 and post-MCV2 antibody concentrations, respectively.
Extended Data Fig. 9 Antibody responses and population immunity dynamics following different measles vaccination schedules.
In (a) and (b), the lines and shaded area are based on 10,000 simulated datasets. (a) Antibody responses (mIU/ml) and (b) the proportion of children with above-threshold (120 mIU/ml) concentration, stratified by the age at dose 1. The solid line represents the median of the individual mean concentrations (a) or the proportion of protected individuals (b) based on the simulated observations from children receiving a two-dose schedule (that is, dose 1 at 6/8/12 months and dose 2 at 15/24 months), while the dotted line denotes those from children receiving a single-dose schedule (that is, dose 1 at 6/8/12 months). The colour of shaded area in (a) from light to darkness reflects the 2.5 and 97.5 percentiles and the interquartile range of the individual mean concentration from the simulated individuals’ antibody responses, whereas it represents 95% confidence intervals in (b).
Extended Data Fig. 10 Estimated population immunity dynamics across different measles vaccination schedules, using a protective threshold of 200 mIU/ml.
The lines and shaded areas are based on 10,000 simulated datasets. The solid and dotted line represents the proportion of protected individuals based on the simulated observations from children receiving a two-dose schedule (that is, dose 1 at 6/8/12 months and dose 2 at 18 months) or a single-dose schedule. The colour of the shaded area represents 95% confidence intervals.
Supplementary information
Supplementary Information
Supplementary Tables 1–9 and Figs. 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., O’Driscoll, M., Wang, Q. et al. Dynamics of measles immunity from birth and following vaccination. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01694-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41564-024-01694-x