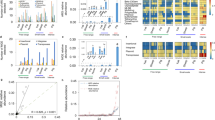
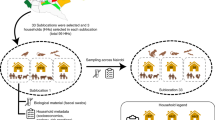
Abstract
Globally, half a billion people are employed in animal agriculture and are directly exposed to the associated microorganisms. However, the extent to which such exposures affect resident human microbiomes is unclear. Here we conducted a longitudinal profiling of the nasal and faecal microbiomes of 66 dairy farmers and 166 dairy cows over a year-long period. We compare farmer microbiomes to those of 60 age-, sex- and ZIP code-matched people with no occupational exposures to farm animals (non-farmers). We show that farming is associated with microbiomes containing livestock-associated microbes; this is most apparent in the nasal bacterial community, with farmers harbouring a richer and more diverse nasal community than non-farmers. Similarly, in the gut microbial communities, we identify more shared microbial lineages between cows and farmers from the same farms. Additionally, we find that shared microbes are associated with antibiotic resistance genes. Overall, our study demonstrates the interconnectedness of human and animal microbiomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All 16S and shotgun sequencing data pertaining to this study are available from the NCBI SRA under BioProject ID PRJNA964705. The databases used in this study include the SILVA database (v.138.1) (https://www.arb-silva.de/documentation/release-1381/), DeconSeq (v.4.3; -dbs hsref38,cow) (https://deconseq.sourceforge.net/), shortBRED (v.0.9.4) (https://github.com/biobakery/biobakery/wiki/shortbred), CARD (v.3.2.2) (https://card.mcmaster.ca/download), NCBI AR gene catalogue (v.2022-04-04.1) (https://www.ncbi.nlm.nih.gov/pathogens/refgene/#), UniRef90 (v.2022-05-29) (https://ftp.uniprot.org/pub/databases/uniprot/uniref/uniref90) and BLASTn (blast-plus) (https://blast.ncbi.nlm.nih.gov/doc/blast-help/downloadblastdata.html#downloadblastdata). Source data are provided with this paper.
Code availability
The code for all computational analyses is available at https://github.com/dantaslab/DOME/tree/main/Scripts.
References
Moving Towards Sustainability: The Livestock Sector and the World Bank (The World Bank, 2020); https://www.worldbank.org/en/topic/agriculture/brief/moving-towards-sustainability-the-livestock-sector-and-the-world-bank
Farming and Farm Income (US Department of Agriculture, 2023); https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/farming-and-farm-income/
Sowiak, M. et al. An assessment of potential exposure to bioaerosols among swine farm workers with particular reference to airborne microorganisms in the respirable fraction under various breeding conditions. Aerobiologia 28, 121–133 (2012).
Stein, M. M. et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N. Engl. J. Med. 375, 411–421 (2016).
Levy, S. B., FitzGerald, G. B. & Macone, A. B. Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature 260, 40–42 (1976).
Angen, O. et al. Transmission of methicillin-resistant Staphylococcus aureus to human volunteers visiting a swine farm. Appl. Environ. Microbiol. 83, e01489-17 (2017).
Depner, M. et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 26, 1766–1775 (2020).
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364, 701–709 (2011).
Illi, S. et al. Protection from childhood asthma and allergy in Alpine farm environments—the GABRIEL advanced studies. J. Allergy Clin. Immunol. 129, 1470–1477 (2012).
Steiman, C. A. et al. Patterns of farm exposure are associated with reduced incidence of atopic dermatitis in early life. J. Allergy Clin. Immunol. 146, 1379–1386 (2020).
Chen, D. et al. Campylobacter colonization, environmental enteric dysfunction, stunting and associated risk factors among young children in rural Ethiopia: a cross-sectional study from the Campylobacter Genomics and Environmental Enteric Dysfunction (CAGED) Project. Front. Public Health 8, 615793 (2020).
Jenkins, P. L., Earle-Richardson, G., Bell, E. M., May, J. J. & Green, A. Chronic disease risk in central New York dairy farmers: results from a large health survey 1989-1999. Am. J. Ind. Med. 47, 20–26 (2005).
Eduard, W., Douwes, J., Omenaas, E. & Heederik, D. Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax 59, 381–386 (2004).
Carnes, M. U. et al. House dust endotoxin levels are associated with adult asthma in a US farming population. Ann. Am. Thorac. Soc. 14, 324–331 (2017).
Omland, O., Hjort, C., Pedersen, O. F., Miller, M. R. & Sigsgaard, T. New-onset asthma and the effect of environment and occupation among farming and nonfarming rural subjects. J. Allergy Clin. Immunol. 128, 761–765 (2011).
Radon, K., Schulze, A. & Nowak, D. Inverse association between farm animal contact and respiratory allergies in adulthood: protection, underreporting or selection? Allergy 61, 443–446 (2006).
Portengen, L., Preller, L., Tielen, M., Doekes, G. & Heederik, D. Endotoxin exposure and atopic sensitization in adult pig farmers. J. Allergy Clin. Immunol. 115, 797–802 (2005).
Smit, L. A. et al. Exposure–response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur. Respir. J. 31, 1241–1248 (2008).
Fisher, J. A. et al. Residential proximity to intensive animal agriculture and risk of lymphohematopoietic cancers in the agricultural health study. Epidemiology 31, 478–489 (2020).
t′Mannetje, A., Eng, A. & Pearce, N. Farming, growing up on a farm and haematological cancer mortality. Occup. Environ. Med. 69, 126–132 (2012).
Van Boeckel, T. P. et al. Global trends in antimicrobial use in food animals. Proc. Natl Acad. Sci. USA 112, 5649–5654 (2015).
Van Boeckel, T. P. et al. Reducing antimicrobial use in food animals. Science 357, 1350–1352 (2017).
Van Boeckel, T. P. et al. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 365, eaaw1944 (2019).
Pitta, D. W. et al. The distribution of microbiomes and resistomes across farm environments in conventional and organic dairy herds in Pennsylvania. Environ. Microbiome 15, 21 (2020).
Mulchandani, R., Wang, Y., Gilbert, M. & Van Boeckel, T. P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 3, e0001305 (2023).
Van Den Broek, I. V. F. et al. Methicillin-resistant Staphylococcus aureus in people living and working in pig farms. Epidemiol. Infect. 137, 700–708 (2009).
Pirolo, M. et al. Unidirectional animal-to-human transmission of methicillin-resistant Staphylococcus aureus ST398 in pig farming; evidence from a surveillance study in southern Italy. Antimicrob. Resist. Infect. Control 8, 187 (2019).
Garcia-Graells, C. et al. Dynamic of livestock-associated methicillin-resistant Staphylococcus aureus CC398 in pig farm households: a pilot study. PLoS ONE 8, e65512 (2013).
Cuny, C. et al. Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS ONE 4, e6800 (2009).
Larsen, J. et al. Meticillin-resistant Staphylococcus aureus CC398 is an increasing cause of disease in people with no livestock contact in Denmark, 1999 to 2011. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2015.20.37.30021 (2015).
Liu, C. M. et al. Escherichia coli ST131-H22 as a foodborne uropathogen. mBio 9, e00470-18 (2018).
Kuthyar, S. & Reese, A. T. Variation in microbial exposure at the human-animal interface and the implications for microbiome-mediated health outcome. mSystems 6, e0056721 (2021).
Sun, J. et al. Environmental remodeling of human gut microbiota and antibiotic resistome in livestock farms. Nat. Commun. 11, 1427 (2020).
Kraemer, J. G., Aebi, S., Hilty, M. & Oppliger, A. Nasal microbiota composition dynamics after occupational change in animal farmers suggest major shifts. Sci. Total Environ. 782, 146842 (2021).
Kraemer, J. G., Ramette, A., Aebi, S., Oppliger, A. & Hilty, M. Influence of pig farming on the human nasal microbiota: key role of airborne microbial communities. Appl. Environ. Microbiol. 84, e02470-17 (2018).
Abreu, N. A. et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 4, 151ra124 (2012).
VanWormer, J. J., Bendixsen, C. G. & Shukla, S. K. Dairy farm work and protection from gastrointestinal illness. J. Agromed 28, 640–646 (2023).
Blanco-Miguez, A. et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 41, 1633–1644 (2023).
Beghini, F. et al. Integrating taxonomic, functional and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 10, e65088 (2021).
Antharam, V. C. et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 51, 2884–2892 (2013).
Olm, M. R. et al. inStrain profiles population microdiversity from metagenomic data and sensitively detects shared microbial strains. Nat. Biotechnol. 39, 727–736 (2021).
Manara, S. et al. Microbial genomes from non-human primate gut metagenomes expand the primate-associated bacterial tree of life with over 1,000 novel species. Genome Biol. 20, 299 (2019).
Mahmud, B., Boolchandani, M., Patel, S. & Dantas, G. Functional metagenomics to study antibiotic resistance. Methods Mol. Biol. 2601, 379–401 (2023).
Forsberg, K. J. et al. The shared antibiotic resistome of soil bacteria and human pathogens. Science 337, 1107–1111 (2012).
Gibson, M. K. et al. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat. Microbiol. 1, 16024 (2016).
Gasparrini, A. J. et al. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 4, 2285–2297 (2019).
Campbell, T. P. et al. The microbiome and resistome of chimpanzees, gorillas, and humans across host lifestyle and geography. ISME J. 14, 1584–1599 (2020).
2021 Summary Report on Antimicrobials Sold or Distributed for Use in Food-producing Animals (US Food and Drug Administration, 2022); https://www.fda.gov/media/163739/download
Alcock, B. P. et al. CARD 2023: expanded curation, support for machine learning and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 51, D690–D699 (2023).
Feldgarden, M. et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response and virulence. Sci. Rep. 11, 12728 (2021).
Anthony, W. E. et al. Acute and persistent effects of commonly used antibiotics on the gut microbiome and resistome in healthy adults. Cell Rep. 39, 110649 (2022).
de Nies, L. et al. Evolution of the murine gut resistome following broad-spectrum antibiotic treatment. Nat. Commun. 13, 2296 (2022).
Johnson, T. A. et al. Clusters of antibiotic resistance genes enriched together stay together in swine agriculture. mBio 7, e02214–e02215 (2016).
Xihui, Z. et al. Antibiotic resistance of Riemerella anatipestifer and comparative analysis of antibiotic-resistance gene detection methods. Poult. Sci. 102, 102405 (2023).
Fenske, G. J., Ghimire, S., Antony, L., Christopher-Hennings, J. & Scaria, J. Integration of culture-dependent and independent methods provides a more coherent picture of the pig gut microbiome. FEMS Microbiol. Ecol. 96, fiaa022 (2020).
Cusco, A., Perez, D., Vines, J., Fabregas, N. & Francino, O. Novel canine high-quality metagenome-assembled genomes, prophages and host-associated plasmids provided by long-read metagenomics together with Hi-C proximity ligation. Micro. Genom. 8, 000802 (2022).
Brito, I. L. et al. Mobile genes in the human microbiome are structured from global to individual scales. Nature 535, 435–439 (2016).
Wallace, M. J., Jean, S., Wallace, M. A., Burnham, C. D. & Dantas, G. Comparative genomics of Bacteroides fragilis group isolates reveals species-dependent resistance mechanisms and validates clinical tools for resistance prediction. mBio 13, e0360321 (2022).
Modi, S. R., Lee, H. H., Spina, C. S. & Collins, J. J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499, 219–222 (2013).
Hernando-Amado, S., Coque, T. M., Baquero, F. & Martinez, J. L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 4, 1432–1442 (2019).
Kieke, A. L. et al. Validation of health event capture in the Marshfield Epidemiologic Study Area. Clin. Med. Res. 13, 103–111 (2015).
Sukhum, K. V. et al. Manure microbial communities and resistance profiles reconfigure after transition to manure pits and differ from those in fertilized field soil. mBio 12, e00798-21 (2021).
D’Souza, A. W. et al. Cotrimoxazole prophylaxis increases resistance gene prevalence and α-diversity but decreases β-diversity in the gut microbiome of Human Immunodeficiency Virus-exposed, uninfected infants. Clin. Infect. Dis. 71, 2858–2868 (2020).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Davis, N. M., Proctor, D. M., Holmes, S. P., Relman, D. A. & Callahan, B. J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 226 (2018).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003).
Lloyd-Price, J. et al. Erratum: Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 551, 256 (2017).
Staff, P. O. Correction: Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS ONE 10, e0131262 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Schmieder, R. & Edwards, R. Fast identification and removal of sequence contamination from genomic and metagenomic datasets. PLoS ONE 6, e17288 (2011).
Li, D., Liu, C. M., Luo, R., Sadakane, K. & Lam, T. W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Wu, Y. W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607 (2016).
Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Alneberg, J. et al. Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146 (2014).
Sieber, C. M. K. et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 3, 836–843 (2018).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells and metagenomes. Genome Res. 25, 1043–1055 (2015).
Chaumeil, P. A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 36, 1925–1927 (2019).
Schwengers, O. et al. Bakta: rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Micro. Genom. 7, 000685 (2021).
Olm, M. R., Brown, C. T., Brooks, B. & Banfield, J. F. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Zankari, E. et al. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644 (2012).
Feldgarden, M. et al. Validating the AMRFinder Tool and Resistance Gene Database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 63, e00483-19 (2019).
Gibson, M. K., Forsberg, K. J. & Dantas, G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J. 9, 207–216 (2015).
Kaminski, J. et al. High-specificity targeted functional profiling in microbial communities with ShortBRED. PLoS Comput. Biol. 11, e1004557 (2015).
Moore, A. M. et al. Pediatric fecal microbiota harbor diverse and novel antibiotic resistance genes. PLoS ONE 8, e78822 (2013).
Moore, A. M. et al. Gut resistome development in healthy twin pairs in the first year of life. Microbiome 3, 27 (2015).
Pehrsson, E. C. et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature 533, 212–216 (2016).
Forsberg, K. J. et al. Bacterial phylogeny structures soil resistomes across habitats. Nature 509, 612–616 (2014).
Clemente, J. C. et al. The microbiome of uncontacted Amerindians. Sci. Adv. 1, e1500183 (2015).
Tsukayama, P. et al. Characterization of wild and captive baboon gut microbiota and their antibiotic resistomes. mSystems 3, e00016–e00018 (2018).
Suzek, B. E., Huang, H., McGarvey, P., Mazumder, R. & Wu, C. H. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics 23, 1282–1288 (2007).
Jain, C., Rodriguez, R, L., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9, 5114 (2018).
Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17, e1009442 (2021).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Acknowledgements
We thank all study participants, particularly the dairy farmers and dairy-farm owners, who, in spite of their busy schedules and harvesting season, participated in the study and also allowed their cows to be enrolled in the study. We thank and acknowledge R. Pilsner, M. Presson and N. Esser of Marshfield Agricultural Station for helping with biospecimen collection from dairy farms. We would like to acknowledge the IACUC of the University of Wisconsin-Madison for reviewing our animal protocol and guidance. We also thank the staff at The Edison Family Center for Genome Sciences & Systems Biology at the Washington University School of Medicine in St Louis, including E. Martin and B. Koebbe for computational support, J. Hoisington-López and M. Crosby for managing the high-throughput sequencing core, and B. Dee, K. Matheny, J. Theodore and K. Page for administrative support. Finally, we would like to thank the members of the Dantas laboratory for helpful general discussions and comments on the manuscript. We thank the National Institute for Occupational Safety and Health of the US Centers for Disease Control and Prevention (grant no. R01OH011578 to G.D. and S.K.S.) for funding support, as well as Marshfield Clinic Research Institute and Weber Endowment Fund (to S.K.S.), the Society for Healthcare Epidemiology of America Research Scholar Award (to K.V.S.), the Initiative for Maximizing Student Development R25 (grant no. GM103757 to R.C.V.) and the Genome Analysis Training Program T32 (grant no. HG000045 to R.C.V.).
Author information
Authors and Affiliations
Contributions
S.K.S., G.D., C.G.B. and J.J.V. designed the study and secured the funding. S.K.S., G.D., C.G.B., J.J.V. and K.K. strategized the sample collection method. T.K., T.L. and E.K. performed the logistics of sample collection, processing and management of the sample databases. B.M., R.C.V., K.V.S., S.P. and J.L. processed the samples and prepared the sequencing libraries. B.M., R.C.V., L.R.H. and A.K. conducted the computational analysis. B.M. wrote the initial draft of the manuscript, with subsequent review and editing by R.C.V., L.R.H., C.G.B., J.J.V., S.K.S. and G.D. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Timothy Walsh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Genus-level richness of nasal and faecal samples.
Genus-level richness of the cow, farmer, and non-farmer nasal (a) and faecal (b) microbiomes across seasons. Boxplots show median (center line), quartiles (box limits), and 1.5x interquartile range (whiskers). Dots correspond to individual samples. Half-violins show the data distribution. P values were calculated using the two-tailed Wilcoxon rank-sum test, with subsequent Benjamini-Hochberg correction for multiple hypotheses.
Extended Data Fig. 2 Microbial families differentially abundant in nasal samples.
Microbial families with significant (P < 0.05) differential abundances between farmer (n = 145) and non-farmer (n = 103) nasal microbiomes are indicated. For each family, when significant, the corresponding coefficient in cow samples (n = 363) relative to those of non-farmers is also shown. Points denote mean coefficients; whiskers correspond to standard error. Enrichment tested using MaAsLin 2 (see Methods), Benjamini-Hochberg correction for multiple hypotheses.
Extended Data Fig. 3 Principal coordinate analysis of Bray-Curtis dissimilarities.
Analysis consists of genus (a–h) and species (i-l) compositions of human fecal samples across seasons. Taxonomic profiling is based on 16S (a-d) and shotgun metagenomic (e-l) sequencing data P values were calculated using PERMANOVA and adjusted for multiple hypotheses using the Benjamini-Hochberg method.
Extended Data Fig. 4 Average fecal Bray-Curtis distance of farmers and non-farmers to cows residing in the same or different collection site.
Beta diversities are based on genus (a) or resistome (b) compositions. Boxplots show median (center line), quartiles (box limits), and 1.5x interquartile range (whiskers). Dots correspond to individual samples. Half-violins show the data distribution. P values were calculated using the two-tailed Wilcoxon rank-sum test, with subsequent Benjamini-Hochberg correction for multiple hypotheses. No significant differences (P < 0.05) were identified.
Extended Data Fig. 5 Differential abundances of microbiome characteristics across farmers and non-farmers.
Species (a), genera (b), and microbial pathways (c) with significant (P < 0.05) differential abundances between farmer (n = 134) and non-farmer (n = 95) fecal microbiomes are indicated. Points denote mean coefficients; whiskers correspond to standard error. Enrichment tested using MaAsLin 2 (see Methods), Benjamini-Hochberg correction for multiple hypotheses.
Extended Data Fig. 6 Total ARG relative abundances of cow, farmer, and non-farmer fecal microbiomes across seasons.
Boxplots show median (center line), quartiles (box limits), and 1.5x interquartile range (whiskers). Dots correspond to individual samples. Half-violins show the data distribution. P values were calculated using the two-tailed Wilcoxon rank-sum test, with subsequent Benjamini-Hochberg correction for multiple hypotheses.
Extended Data Fig. 7 ARGs overrepresented in the cow gut.
ARGs enriched (P < 0.05) in the cow gut resistome (n = 330) relative to that of humans (n = 229). The enrichment coefficients were determined through MaAsLin2 (see Methods), with Benjamini-Hochberg correction for multiple hypotheses. The ARGs are colored according to the corresponding antibiotic classes.
Extended Data Fig. 8 Farmer and non-farmer gut ARG richness in spring.
The analysis of richness involved only ARGs correlated with genera that i) are enriched in the farmer and cow nasal microbiomes relative to that of non-farmers, and ii) represent the microbial lineages cooccurring the farmer and cow guts. Boxplots show median (center line), quartiles (box limits), and 1.5x interquartile range (whiskers). Dots correspond to individual samples. Half-violins show the data distribution. The P value was calculated using the two-tailed Wilcoxon rank-sum test.
Extended Data Fig. 9 Rarefaction analysis for 16S rRNA sequencing.
Analysis consisted of fecal (a,c,e) and nasal (b,d,f) samples of cows (a, b), farmers (c, d), and non-farmers (e, f). The analysis was based on genus richness. Boxplots show median (center line), quartiles (box limits), and 1.5x interquartile range (whiskers). Dots correspond to individual subsamples. Half-violins show the data distribution. The differences among subsamples were tested for significance using Dunn’s test, and the P values adjusted for multiple hypotheses using Benjamini-Hochberg. ns, not significant.
Extended Data Fig. 10 Rarefaction analysis for shotgun sequencing.
Analysis consisted of cow (a), farmer (b), and non-farmer (c) fecal samples. The analysis was based on ARG richness. Boxplots show median (center line), quartiles (box limits), and 1.5x interquartile range (whiskers). Dots correspond to individual subsamples. Half-violins show the data distribution. The differences among subsamples were tested for significance using Dunn’s test, and the P values adjusted for multiple hypotheses using Benjamini-Hochberg. ns, not significant.
Supplementary information
Supplementary Information
Information guide to Supplementary Tables 1–8.
Supplementary Tables 1–8
Supplementary Tables 1–8.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahmud, B., Vargas, R.C., Sukhum, K.V. et al. Longitudinal dynamics of farmer and livestock nasal and faecal microbiomes and resistomes. Nat Microbiol 9, 1007–1020 (2024). https://doi.org/10.1038/s41564-024-01639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01639-4