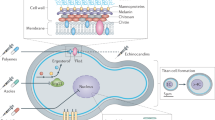
Abstract
Antibiotic tolerance is the ability of a susceptible population to survive high doses of cidal drugs and has been shown to compromise therapeutic outcomes in bacterial infections. In comparison, whether fungicide tolerance can be induced by host-derived factors during fungal diseases remains largely unknown. Here, through a systematic evaluation of metabolite–drug–fungal interactions in the leading fungal meningitis pathogen, Cryptococcus neoformans, we found that brain glucose induces fungal tolerance to amphotericin B (AmB) in mouse brain tissue and patient cerebrospinal fluid via the fungal glucose repression activator Mig1. Mig1-mediated tolerance limits treatment efficacy for cryptococcal meningitis in mice via inhibiting the synthesis of ergosterol, the target of AmB, and promoting the production of inositolphosphorylceramide, which competes with AmB for ergosterol. Furthermore, AmB combined with an inhibitor of fungal-specific inositolphosphorylceramide synthase, aureobasidin A, shows better efficacy against cryptococcal meningitis in mice than do clinically recommended therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All sequencing data are archived on GEO (accession: GSE188965). All other data needed to evaluate the conclusions are available within the Article or its Supplementary Information. Source data are provided with this paper. Any additional data are available from the corresponding author.
References
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv113 (2012).
Iyer, K. R., Revie, N. M., Fu, C., Robbins, N. & Cowen, L. E. Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat. Rev. Microbiol. 19, 454–466 (2021).
Cavassin, F. B., Baú-Carneiro, J. L., Vilas-Boas, R. R. & Queiroz-Telles, F. Sixty years of amphotericin B: an overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 10, 115–147 (2021).
Chen, L. et al. Confronting antifungal resistance, tolerance, and persistence: advances in drug target discovery and delivery systems. Adv. Drug Deliv. Rev. 200, 115007 (2023).
Rodero, L. et al. Timed-kill curves for Cryptococcus neoformans isolated from patients with AIDS. Med. Mycol. 38, 201–207 (2000).
Córdoba, S., Vivot, W., Szusz, W., Isla, G. & Davel, G. Comparison of different in vitro tests to detect Cryptococcus neoformans not susceptible to amphotericin B. Mycopathologia 179, 359–371 (2015).
Smith, K. D. et al. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 59, 7197–7204 (2015).
Davey, K. G., Holmes, A. D., Johnson, E. M., Szekely, A. & Warnock, D. W. Comparative evaluation of FUNGITEST and broth microdilution methods for antifungal drug susceptibility testing of Candida species and Cryptococcus neoformans. J. Clin. Microbiol. 36, 926–930 (1998).
Chiu, Y. S. et al. Survey of amphotericin B susceptibility of Candida clinical isolates determined by Etest. J. Microbiol. Immunol. Infect. 39, 335–341 (2006).
Rosenberg, A. et al. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat. Commun. 9, 2470 (2018).
Berman, J. & Krysan, D. J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 18, 319–331 (2020).
Brauner, A., Fridman, O., Gefen, O. & Balaban, N. Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 14, 320–330 (2016).
Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5, 48–56 (2007).
Vahedi-Shahandashti, R. et al. Aspergillus terreus and the interplay with amphotericin B: from resistance to tolerance? Antimicrob. Agents Chemother. 66, e0227421 (2022).
Barchiesi, F. et al. Tolerance to amphotericin B in clinical isolates of Candida tropicalis. Diagn. Microbiol. Infect. Dis. 50, 179–185 (2004).
Balaban, N. Q. et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 17, 441–448 (2019).
Shestov, A. A. et al. Simultaneous measurement of glucose transport and utilization in the human brain. Am. J. Physiol. Endocrinol. Metab. 301, E1040–E1049 (2011).
Tsai, S. T., Lin, F. Y., Chen, P. S., Chiang, H. Y. & Kuo, C. C. Three-year mortality in cryptococcal meningitis: hyperglycemia predict unfavorable outcome. PLoS ONE 16, e0251749 (2021).
de Azambuja, A. Z., Wissmann Neto, G., Watte, G., Antoniolli, L. & Goldani, L. Z. Cryptococcal meningitis: a retrospective cohort of a Brazilian reference hospital in the post-HAART era of universal access. Can. J. Infect. Dis. Med. Microbiol. 2018, 6512468 (2018).
Marincu, I. et al. Clinical profile of 24 AIDS patients with cryptococcal meningitis in the HAART era: a report from an infectious diseases tertiary hospital in western Romania. Diagnostics 12, 54 (2021).
Meya, D. B. et al. Cellular immune activation in cerebrospinal fluid from Ugandans with cryptococcal meningitis and immune reconstitution inflammatory syndrome. J. Infect. Dis. 211, 1597–1606 (2015).
Conrad, M. et al. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 38, 254–299 (2014).
Johnston, M. Feasting, fasting and fermenting. Glucose sensing in yeast and other cells. Trends Genet. 15, 29–33 (1999).
Vankuyk, P. A. et al. Aspergillus niger mstA encodes a high-affinity sugar/H+ symporter which is regulated in response to extracellular pH. Biochem. J. 379, 375–383 (2004).
Cupertino, F. B., Virgilio, S., Freitas, F. Z., de Souza Candido, T. & Bertolini, M. C. Regulation of glycogen metabolism by the CRE-1, RCO-1 and RCM-1 proteins in Neurospora crassa. The role of CRE-1 as the central transcriptional regulator. Fungal Genet. Biol. 77, 82–94 (2015).
Caza, M., Hu, G., Price, M., Perfect, J. R. & Kronstad, J. W. The zinc finger protein Mig1 regulates mitochondrial function and azole drug susceptibility in the pathogenic fungus Cryptococcus neoformans. mSphere 1, e00080–15 (2016).
De Vit, M. J., Waddle, J. A. & Johnston, M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol. Biol. Cell 8, 1603–1618 (1997).
Kodedová, M. & Sychrová, H. Synthetic antimicrobial peptides of the halictines family disturb the membrane integrity of Candida cells. Biochim. Biophys. Acta Biomembr. 1859, 1851–1858 (2017).
Yang, X. et al. Study on the inhibitory activity and possible mechanism of myriocin on clinically relevant drug-resistant Candida albicans and its biofilms. Biol. Pharm. Bull. 44, 305–315 (2021).
Sharma, S. et al. Sphingolipid biosynthetic pathway genes FEN1 and SUR4 modulate amphotericin B resistance. Antimicrob. Agents Chemother. 58, 2409–2414 (2014).
Palacios, D. S., Dailey, I., Siebert, D. M., Wilcock, B. C. & Burke, M. D. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl Acad. Sci. USA 108, 6733–6738 (2011).
Marques, J. T., Marinho, H. S. & de Almeida, R. F. M. Sphingolipid hydroxylation in mammals, yeast and plants – an integrated view. Prog. Lipid Res. 71, 18–42 (2018).
Klose, C. et al. Yeast lipids can phase-separate into micrometer-scale membrane domains. J. Biol. Chem. 285, 30224–30232 (2010).
Li, J. et al. Large-scaled human serum sphingolipid profiling by using reversed-phase liquid chromatography coupled with dynamic multiple reaction monitoring of mass spectrometry: method development and application in hepatocellular carcinoma. J. Chromatogr. A 1320, 103–110 (2013).
Salem, H. F., Ahmed, S. M., Hassaballah, A. E. & Omar, M. M. Targeting brain cells with glutathione-modulated nanoliposomes: in vitro and in vivo study. Drug Des. Devel. Ther. 9, 3705–3727 (2015).
Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living with HIV (World Health Organization, 2022).
Takesako, K. et al. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J. Antibiot. 46, 1414–1420 (1993).
Rauseo, A. M., Coler-Reilly, A., Larson, L. & Spec, A. Hope on the horizon: novel fungal teatments in development. Open Forum Infect. Dis. 7, ofaa016 (2020).
Rollin-Pinheiro, R. et al. Sphingolipid inhibitors as an alternative to treat candidiasis caused by fluconazole-resistant strains. Pathogens 10, 856 (2021).
Wuts, P. G. et al. Generation of broad-spectrum antifungal drug candidates from the natural product compound aureobasidin A. ACS Med. Chem. Lett. 6, 645–649 (2015).
Wang, L., Zhai, B. & Lin, X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 8, e1002765 (2012).
Fan, Y. & Lin, X. Multiple applications of a transient CRISPR-Cas9 coupled with electroporation (TRACE) system in the Cryptococcus neoformans species complex. Genetics 208, 1357–1372 (2018).
Lee, U., Garcia, T. P., Carroll, R. J., Gilbreath, K. R. & Wu, G. Analysis of repeated measures data in nutrition research. Front. Biosci. (Landmark edn) 24, 1377–1389 (2019).
Ke, W. et al. A forkhead transcription factor contributes to the regulatory differences of pathogenicity in closely related fungal pathogens. mLife 1, 79–91 (2022).
Vanherp, L. et al. Sensitive bioluminescence imaging of fungal dissemination to the brain in mouse models of cryptococcosis. Dis. Model. Mech. 12, dmm039123 (2019).
Taj-Aldeen, S. J. et al. Molecular analysis of resistance and detection of non-wild-type strains using Etest epidemiological cutoff values for amphotericin B and echinocandins for bloodstream Candida infections from a tertiary hospital in Qatar. Antimicrob. Agents Chemother. 62, e00214-18 (2018).
Al-Odaini, N. et al. In vitro antifungal susceptibility profiles of Cryptococcus neoformans var. grubii and Cryptococcus gattii clinical isolates in Guangxi, southern China. Front. Microbiol. 12, 708280 (2021).
Rossato, L. et al. In vitro combination between antifungals and diphenyl diselenide against Cryptococcus species. Mycoses 62, 508–512 (2019).
Saini, N. K. et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat. Microbiol. 1, 16133 (2016).
Tian, X. et al. Cryptococcus neoformans sexual reproduction is controlled by a quorum sensing peptide. Nat. Microbiol. 3, 698–707 (2018).
Maere, S., Heymans, K. & Kuiper, M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21, 3448–3449 (2005).
Brotherton, M. C. et al. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 4, 126–132 (2014).
Lam, S. M. et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 3, 909–922 (2021).
Lam, S. M., Wang, Z., Li, J., Huang, X. & Shui, G. Sequestration of polyunsaturated fatty acids in membrane phospholipids of Caenorhabditis elegans dauer larva attenuates eicosanoid biosynthesis for prolonged survival. Redox Biol. 12, 967–977 (2017).
Sun, X. et al. Sterol C-22 desaturase ERG5 mediates the sensitivity to antifungal azoles in Neurospora crassa and Fusarium verticillioides. Front. Microbiol. 4, 127 (2013).
Wu, J. et al. Apamin-mediated actively targeted drug delivery for treatment of spinal cord injury: more than just a concept. Mol. Pharm. 11, 3210–3222 (2014).
Acknowledgements
We thank members of the Wang lab, and Y. Zhao and E. Yang for sharing constructs and protocols and scientific discussions; Y. Wang for providing the Rhizopus arrhizus strains; T. Liu for providing C. neoformans H99 strain harbouring PH3-GFP-NOP1; G. Liu for quantification of glucose in CSF; B. Shi for lipidomic analysis; L. Su for the time-lapse experiment; and T. Zhao for intracellular ROS measurement. This work was supported by the National Key Research and Development Program (2022YFC2303000 and 2021YFC230000, L.W.; 2021YFA0911300, P.H.; and 2021YFC2100600, X.T.; http://www.most.gov.cn/); the National Natural Science Foundation of China (32100153, P.H.; 31970077, G.-j.H.; 82172291, M.C.; and 82073789, C.L.; http://www.nsfc.gov.cn/); and the CAS Interdisciplinary Innovation Team (L.W.; http://www.cas.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the data analysis. L.C. and L.W outlined the study; L.C., X.T., L.Z., W.W., P.H., Z.M., Y.L., S.L, Z.S., X.F., L.Y., W.K., Y.W., G.S., M.X., G.-j.H., Y.Y., F.B., G.L., M.C., W.F., X.L., C.L. and L.W. designed the experiments. L.C., X.T. and L.Z. conducted most of the studies; L.C. conducted the bioinformatic analysis; L.Z., W.W. and Y.W. analysed the data; M.X., Y.Y., G.S. and M.C. contributed reagents/materials/analysis tools; and L.C., X.T., X.L. and L.W. wrote the manuscript with contributions from other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Glucose at a concentration similar to that in brain tissue is sufficient to induce AmB tolerance.
a, Cells were cultured in glucose-free RPMI medium supplemented with glucose before challenge with 1 hr 10 × MIC AmB treatment. The percent survival was normalized to survival without AmB. Shaded color represents SD of three biological replicates. b, The stimulatory effect of glucose on AmB tolerance is independent of its growth- promoting activity. The influences of different metabolites on the growth of a wildtype C. neoformans strain were evaluated relative to glucose (Down). Yellow dots represent metabolites with similar fungal growth-promoting effect to glucose (0.8–1.2 times CFU to glucose). Histogram indicating the influence of metabolites with similar fungal growth-promoting effect to glucose on the AmB tolerance (Up). Dots are representative of means of two independent experiments. c, AmB MIC levels of the wildtype strain cultured at different glucose concentrations were measured with a method described by CLSI. Yellow indicates fungal cell survival under corresponding drug concentration. d, A minimum duration for killing 99% of cells (MDK99) test of AmB using a wildtype C. neoformans strain treated at 10 × MIC. Cells were cultured on glucose-free RPMI medium supplemented with 0, 0.25, or 4 mM glucose for 6 hrs before exposure to AmB. Data represent mean ± SD (n = 3). Two-tailed unpaired Student’s t test in (d).
Extended Data Fig. 2 Evaluation of the effects of glucose oxidase, MnO2 and gluconic acid on stimulation of AmB tolerance.
a, H2O2 concentrations following GOX-catalyzed glucose conversion and MnO2 treatment were monitored. Data represent the mean ± SD (n = 3). ND, not detected. b, C. neoformans cells were treated at 1 hr 10 × MIC AmB treatment and cultured in YP broth supplemented with 2% glucose or 2% galactose and the noted factors. Data represent the mean ± SD (n = 3).
Extended Data Fig. 3 Glucose, but not its metabolic intermediates, induces AmB tolerance in C. neoformans.
Results from metabolite-AmB screening showed that neither the glucose metabolites generated during glycolysis (left) nor those during the tricarboxylic cycle (right) showed detectable impact on AmB tolerance in wildtype C. neoformans.
Extended Data Fig. 4 Glucose repression (GR) mediates AmB tolerance in C. neoformans.
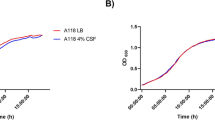
a, Wildtype C. neoformans cells were cultured in media containing 1% glucose and 1% galactose (left), or in media containing only 2% galactose (right). Sugar metabolism was evaluated by HPLC. Cell growth was determined by measuring the absorbance at 600 nm (A600). Data are shown as mean ± SD (n = 3). b, 2-DG, a non-metabolizable analog of glucose, inhibits the growth of C. neoformans when added with an alternative carbon source (galactose) but has no effect on growth when added with a preferred carbon source. c, Percent survival of wildtype cells cultured in media containing 2% galactose or 2% galactose with 0.02% 2-DG upon 10 × MIC AmB exposure for 1 hr. Data are shown as mean ± SD (n = 3). Two-tailed unpaired Student’s t test in (c). Sugar con., sugar concentration; 2-DG, 2-deoxyglucose.
Extended Data Fig. 5 Mig1 plays a critical role in glucose repression and glucose-induced AmB tolerance in C. neoformans.
a, Wildtype and mig1Δ mutant cells were spotted onto the noted YP plates, which were photographed after 3 days. Images are representative of more than three trials. b, Wildtype and mig1Δ mutant cells were cultured in media containing 2% glucose. Cell growth was determined by measuring the absorbance at 600 nm (A600). Data are shown as mean ± SD (n = 3). c, Survival curves of wildtype and mig1Δ cells cultured in the presence of 2% glucose at noted concentration of AmB. Data represent the mean ± SD (n = 3).
Extended Data Fig. 6 The mechanism by which Mig1 affects glucose-induced AmB tolerance revealed by time-series transcriptome analyses.
a, Percent survival of C. neoformans wildtype and mig1Δ cells incubated with AmB at 10 × MIC in YP broth with 2% glucose (Glu) or 2% galactose (Gal). Wildtype and mig1Δ cells with or without AmB treatment for 10, 20, or 30 minutes were collected for further transcriptome analyses. Data represent the mean ± SD (n = 3). b, Principal component analysis (PCA) of dynamic transcriptome data of C. neoformans wildtype and mig1Δ cells treated with AmB (10 × MIC). Each plot represents the mean from three biological repeats. c, Plotting the 7 dimensions determined to be statistically significant by permutation analysis. d, Pearson correlations of metabolite alteration, Mig1 function and AmB treatment to dimensions 1–7. e, Biological functional enrichment analysis of genes specifically regulated by Mig1 in response to glucose. f, Fold change of genes related to ergosterol synthesis under different conditions. Data shown in the figure are from the AmB-untreated samples. Two-sided Wald test in DESeq2 in (f).
Extended Data Fig. 7 Beanplot illustrating relative lipid composition levels of wildtype and mig1Δ cells in the presence of 2% glucose.
PC: phosphatidylcholines; LPC: lyso-PC; IPC: inositolphosphorylceramide; MIPC: mannosyl inositolphosphorylceramide; PhytoCer: phytoceramides; SPH: sphingosine. Two-tailed unpaired Student’s t test.
Extended Data Fig. 8 AmB tolerance is induced by inhibiting ergosterol production and is associated with reduced AmB accumulation.
a, AmB MIC levels of the wildtype cells grown under AmB sensitive conditions (2% galactose) pre-treated with 1 μg/mL terbinafine. b, Relative AmB levels in wildtype and mig1Δ cells cultured on YP medium supplemented with 2% glucose or 2% galactose or 2% galactose with 8 μg/mL terbinafine. Data represent the mean ± SD (n = 3). Two-tailed unpaired Student’s t test in (b).
Extended Data Fig. 9 Pretreatment with AbA does not improve fungicidal efficacy of AmB in the erg6Δ mutant strain.
a, Relative IPC levels of wildtype cells cultured in the presence of 2% glucose with or without AbA treatment (1 μg/mL). Boxplots display the 25th, 50th (median) and 75th quantiles as well as the minimum and maximum values. b, Percent survival of erg6Δ cells cultured in the presence of 2% glucose after 1 hr 10 × MIC AmB treatment with or without AbA (1 μg/mL). Data represent the mean ± SD (n = 3). c, Relative ergosterol levels of wildtype and erg6Δ cells in the presence of 2% glucose. Data represent the mean ± SD (n = 3). ND, not detected.
Extended Data Fig. 10 The potential AmB-potentiating effect of AbA in a mouse model of cryptococcosis.
a, Experimental design of brain infections and treatments of antifungals as noted. Mice were infected intravenously with C. neoformans at day 0. b, Percent survival of wildtype in mouse brains at 7 days after infection, with AmB or AmB-AbA treatment (without liposomal encapsulation). Bars represent the mean ± SD (n = 3). c, Comparison of AbA content in the brain of mice treated with liposome-encapsulated AbA and without liposome-encapsulated AbA as determined by LC-MS/MS. Data represent the mean ± SD (n = 3). d, Relative brain fungal burdens of wildtype at 7 days after infection, with or without treatment with liposome-encapsulated AbA. Data represent the mean ± SD (n = 10). NS, not significant (two-tailed unpaired Student’s t test in (d)). Lipid: liposome encapsulation.
Supplementary information
Supplementary Tables
Supplementary Tables 1–5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Tian, X., Zhang, L. et al. Brain glucose induces tolerance of Cryptococcus neoformans to amphotericin B during meningitis. Nat Microbiol 9, 346–358 (2024). https://doi.org/10.1038/s41564-023-01561-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-023-01561-1
This article is cited by
-
Metabolic sensing tips the balance of drug tolerance in fungal meningitis
Nature Microbiology (2024)
-
Brain glucose mediates amphotericin B tolerance
Nature Reviews Drug Discovery (2024)