Abstract
The host type I interferon (IFN) pathway is a major signature of inflammation induced by the human fungal pathogen, Candida albicans. However, the molecular mechanism for activating this pathway in the host defence against C. albicans remains unknown. Here we reveal that mice lacking cyclic GMP–AMP synthase (cGAS)–stimulator of IFN genes (STING) pathway components had improved survival following an intravenous challenge by C. albicans. Biofilm-associated C. albicans DNA packaged in extracellular vesicles triggers the cGAS–STING pathway as determined by induction of interferon-stimulated genes, IFNβ production, and phosphorylation of IFN regulatory factor 3 and TANK-binding kinase 1. Extracellular vesicle-induced activation of type I IFNs was independent of the Dectin-1/Card9 pathway and did not require toll-like receptor 9. Single nucleotide polymorphisms in cGAS and STING potently altered inflammatory cytokine production in human monocytes challenged by C. albicans. These studies provide insights into the early innate immune response induced by a clinically significant fungal pathogen.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Tsay, S. et al. National burden of candidemia, United States, 2017. Open Forum Infect. Dis. 5, S142 (2018).
Cornely, O. A. et al. Epidemiology and outcome of fungemia in a cancer Cohort Of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin. Infect. Dis. https://doi.org/10.1093/cid/civ293 (2015).
Smeekens, S. P. et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat. Commun. 4, 1342 (2013).
Pekmezovic, M. et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat. Microbiol. 6, 643–657 (2021).
Bourgeois, C. et al. Conventional dendritic cells mount a type I IFN response against Candida spp. Requiring novel phagosomal TLR7-mediated IFN-β signaling. J. Immunol. 186, 3104–3112 (2011).
delFresno, C. et al. Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 38, 1176–1186 (2013).
Jaeger, M. et al. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur. J. Clin. Microbiol. Infect. Dis. 34, 963 (2015).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS–STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Chen, T. et al. The nucleotide receptor STING translocates to the phagosomes to negatively regulate anti-fungal immunity. Immunity 56, 1727–1742.e6 (2023).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Diner, E. J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 3, 1355–1361 (2013).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Fischer, K. et al. Cutibacterium acnes infection induces type I interferon synthesis through the cGAS–STING pathway. Front. Immunol. 11, 2630 (2020).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Hansen, K. et al. Listeria monocytogenes induces IFNβ expression through an IFI16‐, cGAS‐ and STING‐dependent pathway. EMBO J. 33, 1654–1666 (2014).
Neufeldt, C. J. et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS–STING and NF-κB. Commun. Biol. 5, 1–15 (2022).
Iampietro, M. et al. Activation of cGAS/STING pathway upon paramyxovirus infection. iScience 24, 102519 (2021).
Zhang, C. et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398 (2019).
Vargas, G. et al. Compositional and immunobiological analyses ofextracellular vesicles released by Candida albicans. Cell. Microbiol. 17, 389–407 (2015).
Zarnowski, R. et al. Coordination of fungal biofilm development by extracellular vesicle cargo. Nat. Commun. 2021 121 12, 6235 (2021).
Zarnowski, R. et al. A common vesicle proteome drives fungal biofilm development. Proc. Natl Acad. Sci. USA 119, e2211424119 (2022).
Zamith-Miranda, D. et al. Comparative molecular and immunoregulatory analysis of extracellular vesicles from Candida albicans and Candida auris. mSystems 6, e0082221 (2021).
Zarnowski, R. et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. 16, e2006872 (2018).
Erttmann, S. F. et al. The gut microbiota prime systemic antiviral immunity via the cGAS–STING–IFN-I axis. Immunity 55, 847–861.e10 (2022).
Reedy, J. L. et al. The C-type lectin receptor Dectin-2 is a receptor for Aspergillus fumigatus Galactomannan. MBio 14, e0318422 (2023).
Wiesner, D. L. et al. Club cell TRPV4 serves as a damage sensor driving lung allergic inflammation. Cell Host Microbe 27, 614–628.e6 (2020).
Chin, K. C. & Cresswell, P. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl Acad. Sci. USA 98, 15125–15130 (2001).
Kasperkovitz, P. V. et al. Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect. Immun. 79, 4858–4867 (2011).
Gantner, B. N., Simmons, R. M. & Underhill, D. M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277 (2005).
Brown, G. D. & Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 413, 36–37 (2001).
Civril, F. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337 (2013).
Sun, H. et al. A nuclear export signal is required for cGAS to sense cytosolic DNA. Cell Rep. 34, 108586 (2021).
Volkman, H. E., Cambier, S., Gray, E. E. & Stetson, D. B. Tight nuclear tethering of cGAS is essential for preventing autoreactivity. Elife 8, e47491 (2019).
Mosallanejad, K. et al. Three functionally distinct classes of cGAS proteins in nature revealed by self-DNA-induced interferon responses. Preprint at bioRxiv https://doi.org/10.1101/2022.03.09.483681 (2022).
Moreno, J. M., Sanchez-Montero, J. M., Sinisterra, J. V. & Nielsen, L. B. Contribution to the study of the enzymatic activity of Benzonase. J. Mol. Catal. 69, 419–427 (1991).
Rajendran, R. et al. Extracellular DNA release confers heterogeneity in Candida albicans biofilm formation. BMC Microbiol. 14, 303 (2014).
Martins, M. et al. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 169, 323–331 (2010).
Haag, S. M. et al. Targeting STING with covalent small-molecule inhibitors. Nature 559, 269–273 (2018).
Gulen, M. F. et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374–380 (2023).
Dog, S. et al. Single-cell analysis of early antiviral gene expression reveals a determinant of stochastic IFNB1 expression. Integr. Biol. 9, 857 (2017).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Bürckstümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Motwani, M., Pesiridis, S. & Fitzgerald, K. A. DNA sensing by the cGAS–STING pathway in health and disease. Nat. Rev. Genet. 2019 2011 20, 657–674 (2019).
Stawowczyk, M. et al. Pathogenic effects of IFIT2 and interferon-β during fatal systemic Candida albicans infection. mBio 9, e00365–18 (2018).
Zarnowski, R. et al. Coordination of fungal biofilm development by extracellular vesicle cargo. Nat. Commun. 12, 6235 (2021).
Bitencourt, T. A. et al. Fungal extracellular vesicles are involved in intraspecies intracellular communication. mBio 13, e0327221 (2022).
Honorato, L. et al. Extracellular vesicles regulate biofilm formation and yeast-to-hypha differentiation in Candida albicans. mBio 13, e0030122 (2022).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Kanada, M. et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl Acad. Sci. USA 112, 201418401 (2015).
Mack, M. et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6, 769–775 (2000).
Bonsergent, E. et al. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat. Commun. 12, 1–11 (2021).
Balaj, L. et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Al-Nedawi, K. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008).
Coakley, G. et al. Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep. 19, 1545–1557 (2017).
Gomes, M. T. R. et al. STING regulates metabolic reprogramming in macrophages via HIF-1α during Brucella infection. PLoS Pathog. 17, e1009597 (2021).
Zhang, H., Zoued, A., Liu, X., Sit, B. & Waldora, M. K. Type I interferon remodels lysosome function and modifies intestinal epithelial defense. Proc. Natl Acad. Sci. USA 117, 29862–29871 (2020).
Netea, M. G., Brown, G. D., Kullberg, B. J. & Gow, N. A. R. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78 (2008).
Nahum, A., Dadi, H., Bates, A. & Roifman, C. M. The L412F variant of Toll-like receptor 3 (TLR3) is associated with cutaneous candidiasis, increased susceptibility to cytomegalovirus, and autoimmunity. J. Allergy Clin. Immunol. 127, 528–531 (2011).
Ferwerda, G., Meyer-Wentrup, F., Kullberg, B. J., Netea, M. G. & Adema, G. J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 10, 2058–2066 (2008).
Wagener, J. et al. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 10, e1004050 (2014).
Hise, A. G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
Pietrella, D. et al. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur. J. Immunol. 43, 679–692 (2013).
Majer, O. et al. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 8, e1002811 (2012).
Kolben, T. et al. IL-23, IFN-α, and IFN-β in the vaginal fluid of patients suffering from vulvovaginal candidosis. Clin. Exp. Obstet. Gynecol. 44, 7–10 (2017).
Biondo, C. et al. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur. J. Immunol. 41, 1969–1979 (2011).
Han, F. et al. The cGAS-STING signaling pathway contributes to the inflammatory response and autophagy in Aspergillus fumigatus keratitis. Exp. Eye Res. 202, 108366 (2021).
Souza, J. A. M. et al. Characterization of Aspergillus fumigatus extracellular vesicles and their effects on macrophages and neutrophils functions. Front. Microbiol. 10, 2008 (2019).
Rizzo, J. et al. Characterization of extracellular vesicles produced by Aspergillus fumigatus protoplasts. mSphere https://doi.org/10.1128/msphere.00476-20 (2020).
Dutta, O., Espinosa, V., Wang, K., Avina, S. & Rivera, A. Dectin-1 promotes type I and III interferon expression to support optimal antifungal immunity in the lung. Front. Cell Infect. Microbiol. 10, 321 (2020).
Espinosa, V. et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2, eaan5357 (2017).
Jiang, G. L. et al. cGAS knockdown promotes microglial M2 polarization to alleviate neuroinflammation by inhibiting cGAS-STING signaling pathway in cerebral ischemic stroke. Brain Res. Bull. 171, 183–195 (2021).
Tam, J. M. et al. Tetraspanin CD82 organizes Dectin-1 into signaling domains to mediate cellular responses to Candida albicans. J. Immunol. 202, 3256–3266 (2019).
Gray, E. E., Treuting, P. M., Woodward, J. J. & Stetson, D. B. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi–Goutières syndrome. J. Immunol. 195, 1939–1943 (2015).
Barnett, K. C. et al. Phosphoinositide interactions position cGAS at the plasma membrane to ensure efficient distinction between self- and viral DNA. Cell 176, 1432–1446.e11 (2019).
Kasperkovitz, P. V., Cardenas, M. L. & Vyas, J. M. TLR9 Is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J. Immunol. 185, 7614–7622 (2010).
Zamith-Miranda, D., Alves, L. R., Rodrigues, M. L., Nimrichter, L. & Nosanchuk, J. D. Isolation of extracellular vesicles from Candida auris. Methods Mol. Biol. 2517, 173–178 (2022).
Zarnowski, R., Sanchez, H. & Andes, D. R. Large-scale production and isolation of Candida biofilm extracellular matrix. Nat. Protoc. 11, 2320–2327 (2016).
Li, Y. et al. A functional genomics approach to understand variation in cytokine production in humans. Cell 167, 1099–1110.e14 (2016).
Pruim, R. J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
Acknowledgements
We thank all the members of the Vyas and Mansour lab at MGH for useful discussions and reading of the paper. We thank K. Timmer for his expert assistance with all flow cytometry experiments. Financial support: H.B.H. is supported by the Fund for Medical Discovery Fellowship from MGH. J.M.V. is supported by R01AI150181, R01AI136529 and R21AI152499; J.L.R. is supported by NIH/NIAID grant 1K08AI14755; D.R.A. is supported by R01AI073289; R.P.B. is supported by R01AI153405; J.E.N. is supported by R21AI159583 and R01AI145939; J.C.K. is supported by R01AI167993 and R37AI116550; and J.D.N. is supported by R21AI156104.
Author information
Authors and Affiliations
Contributions
Conceptualization: H.B.H. and J.M.V. Investigation: H.B.H., G.K., C.M.R., N.S.K., D.Z.M., R.Z., J.M.T., A.C., C.K.B., L.R., V.K., D.A.V.-B., K.J.B. and J.L.R. Writing: H.B.H., R.A.W. and J.V. Paper review and revision: all authors. Resources: K.M., R.B., J.E.N., F.L.v.d.V., L.R., J.C.K., D.R.A. and J.D.N.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The 500FG cohort was approved by the Arnhem-Nijmegen Medical Ethical Committee (500FG:NL42561.091.12) and performed in accordance with the Declaration of Helsinki. All individuals gave written informed consent to donate venous peripheral blood for research.
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Tobias Hohl, Guilhem Janbon, Mairi Noverr and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
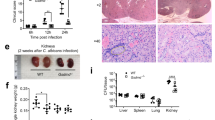
Extended Data Fig. 1 Characterization of organs and serum from C. albicans-infected WT and Sting−/− mice.
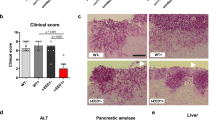
a. Quantification of fungal burden following a C. albicans infection in WT and Sting−/− mice in the kidneys, liver, spleen, and brains (n = 4 for WT and Sting−/− for each time point). Kidney BUN (b) and creatinine levels (c) were measured from serum of infected and uninfected WT and Sting−/− mice over 28 days. n = 5 for each time point. d. Representative gating strategy for flow cytometry analysis showing selection of total kidney cells (SSC v FSC) → live white blood cells (WBC) (7AAD- and CD45 + ) → WBCs that are not B cells or T cells (CD90.2-, CD19-), and of these, how many are neutrophils (Siglec F + , Ly6G + ). e. Quantification of flow-based immunophenotyping of CD45+ WBCs and (f) Siglec F + , Ly6G+ neutrophils from single cell suspensions of kidneys from WT and Sting−/− mice (n = 6 for each group). g. Immunoblot of viperin from kidney homogenates harvested from WT and Sting−/− mice infected with 150,000 C. albicans at day 3 and day 5, compared to the positive control (immortalized WT macrophages stimulated with cGAMP for 6 h prior to lysis). h. Semi-quantification of fungal burden severity in GMS-stained kidney sections of WT and Sting−/− mice (n = 12). Statistical analyses were performed on raw data by means of a non-parametric, two-tailed Mann Whitney U test = 38.5, p = 0.0364. Data are presented as mean values +/- SD.
Extended Data Fig. 2 Further characterization of the viperin and IFNß response to C. albicans EVs.
a. Immunoblot of viperin in lysates from WT macrophages stimulated with PBS, ergosterol alone, EVs from C. albicans or C. auris, or cGAMP. b. IFNß production in the supernatants from the same stimulated WT macrophages as in (a). Significance determined using a one-way ANOVA, ***adj. p = 0.0004, **adj. p = 0.004 c. Average sizes of EVs extracted from C. albicans (grown in different conditions) and C. auris. d. Immunoblot of viperin induction in WT, Card9−/−, Dectin-1−/−, and Tlr9−/− immortalized macrophages following stimulation with PBS or C. albicans EVs. e. IFNß production by WT, cGAS−/−, Sting−/−, Card9−/−, Dectin-1−/−, and Tlr9−/− macrophages following a stimulation with PBS, cGAMP, or C. albicans EVs. Significance determined using a two-way ANOVA and subsequent Dunnett’s multiple comparison test with the exception of Card9−/−, which was an ordinary one-way ANOVA and subsequent Dunnett’s multiple comparison test, *adj. p = 0.0031, **adj. p < 0.01, ***adj. p < 0.001, ****adj. p < 0.0001 vs PBS f. Immunoblot of viperin and g. IFNß production by WT macrophages stimulated with graded amounts of EVs extracted from C. albicans 48 h BF. Significance determined using a one-way ANOVA and subsequent Dunnett’s multiple comparison test *adj. p = 0.0364, ***adj. p = 0.0008, ****adj. p < 0.0001 vs PBS. n = 3 biologically independent samples for all ELISAs and data are presented as mean values +/- SD. All Westerns were done in biological triplicate, representative blot shown. Macrophages were stimulated for 6 h prior to processing.
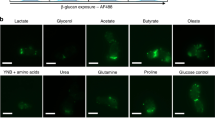
Extended Data Fig. 3 C. albicans EVs induce translocation of cGAS from the nuclear membrane to the cytosol.
Representative microscopy images of macrophages expressing cGAS-GFP and the localization of cGAS following no stimulation or stimulation with DiI only, C. albicans EVs only, or DiI-labeled C. albicans EVs. Macrophages were stimulated for 3 h prior to imaging. Size bar = 5 µm. Microscopy experiments were repeated with appropriate controls in biological triplicate, representative images shown of similar results.
Extended Data Fig. 4 Further characterization of the viperin and IFNß response to C. albicans BF DNA.
a. Immunoblot of viperin induction in WT macrophages stimulated with PBS, C. albicans EVs, Benzonase-treated EVs, and transfected EV DNA. b. Immunoblot of viperin induction and c. IFNβ production in WT macrophages stimulated with DNA extracted from C. albicans biofilms (BF) grown for 48 h, 72 h, 96 h and Lipofectamine reagents only. Significance was determined with an ordinary one-way ANOVA and subsequent Dunnett’s multiple comparison test. **adj. p = 0.0012, ***adj. p = 0.0002, ***adj. p < 0.0001 vs untreated control (Lipofectamine). d. Immunoblot of viperin induction in WT, Sting−/−, Card9−/−, Dectin-1−/−, and Tlr9−/− macrophages following a stimulation with PBS, cGAMP, and DNA extracted from C. albicans BF grown for 72 h. e. IFNß production by WT, cGas−/−, Sting−/−, Card9−/−, Dectin-1−/−, and Tlr9−/− macrophages following a stimulation with PBS and DNA extracted from C. albicans BF grown for 72 h. Significance determined using a two-way ANOVA and subsequent Dunnett’s multiple comparison test. ****p < 0.0001 vs untreated control (PBS). f. Immunoblot of STING pathway component in WT, cGas−/−, and Sting−/− immortalized macrophages treated with increasing concentrations of the STING inhibitor, H-151. Cells were treated with H-151 1 h prior to stimulation with PBS, cGAMP, C. albicans BF DNA, or LPS (STING independent positive control). g. Immunoblot of viperin and actin in WT, cGas−/−, and Sting−/− macrophages treated with the H-151 1 h prior to PBS, cGAMP, C. albicans BF DNA, or C. albicans EV stimulation. All macrophages were stimulated for 6 h prior to processing. n = 3 biologically independent samples for all ELISAs and data are presented as mean values +/- SD. All Westerns were done in biological triplicate, representative blot shown.
Extended Data Fig. 5 Regional association plots for SNPs identified in cGAS and STING.
Regional association plot of SNPs around the MB21D1gene locus for Candida-induced TNF–α concentrations (a) and for Candida-induced IL-6 concentrations (b). Regional association plot of SNPs around the TMEM173 gene locus for Candida- induced TNF–α concentrations (c) and for Candida-induced IL- 6 concentrations (d). The -log10 p-values of imputed SNPs are plotted on the y-axis against their physical position (NCBI build 36) on the x-axis. The most strongly associated SNPs in the regions are represented with purple diamond and surrounding markers are color coded according to their correlation coefficient (r2) with the top SNP using the hg19/1000 Genomes European samples. The light blue lines denote the estimated recombination rates. e. Evidence of the top SNPs regulation the expression levels of MB21D1 and TMEM173. We queried the most significant or top SNPs for statistical evidence of association with the target genes using the eQTLGen database and considered a p value of < 5 × 10−8 to be the threshold for significant cytokine QTLs. f. TNF production by WT, cGas−/−, and Sting−/− murine macrophages following a 2 h co-culture with live C. albicans. ****adj. p < 0.0001 vs. WT. n = 3 biologically independent samples for ELISA and data are presented as mean values +/- SD. Significance determined using an ordinary one-way ANOVA and subsequent Dunnett’s multiple comparison test.
Supplementary information
Supplementary Table 1
Extended Data Table 1. C. albicans genes in BF EVs.
Supplementary Table 2
Extended Data Table 2. Normalized counts of Ncounter host response panel analysis ratios. Genes have been normalized to the controls included in the plate for each genotype to account for variability. Tabs A-B, and E-H includes these normalized ratios counts ± s.d. for each gene. Each NanoString experiment was run in biological duplicate. Data analysis was performed using nSolver 4.0. To account for differences in total RNA per lane, hybridization efficiency and post-hybridization processing, the counts of 773 target RNAs were normalized on the basis of negative controls (background subtraction) and the geometric mean of 12 positive control RNA counts. Fold changes and P values (Benjamini–Yekutieli) were obtained using nSolver Advance Analysis 4.0.
Source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown Harding, H., Kwaku, G.N., Reardon, C.M. et al. Candida albicans extracellular vesicles trigger type I IFN signalling via cGAS and STING. Nat Microbiol 9, 95–107 (2024). https://doi.org/10.1038/s41564-023-01546-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-023-01546-0