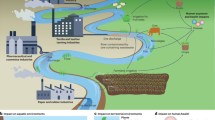
Abstract
Plastics are indispensable in everyday life and industry, but the environmental impact of plastic waste on ecosystems and human health is a huge concern. Microbial biotechnology offers sustainable routes to plastic production and waste management. Bacteria and fungi can produce plastics, as well as their constituent monomers, from renewable biomass, such as crops, agricultural residues, wood and organic waste. Bacteria and fungi can also degrade plastics. We review state-of-the-art microbial technologies for sustainable production and degradation of bio-based plastics and highlight the potential contributions of microorganisms to a circular economy for plastics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Global Plastics Outlook: Policy Scenarios to 2060 (OECD Publishing, 2022).
The New Plastics Economy—Rethinking the Future of Plastics (World Economic Forum, 2016).
Global Plastics Outlook: Greenhouse Gas Emissions from Plastics Lifecycle—Projections (OECD Environment Statistics, 2023).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Global Plastics Outlook: Plastic Leakage to the Aquatic Environments by Region—Projections (OECD Publishing, 2023).
Lim, X. Microplastics are everywhere—but are they harmful? Nature 593, 22–25 (2021).
Shen, M. C. et al. (Micro)plastic crisis: un-ignorable contribution to global greenhouse gas emissions andclimate change. J. Clean. Prod. 254, 120138 (2020).
Hulea, V. Toward platform chemicals from bio-based ethylene: heterogeneous catalysts and processes. ACS Catal. 8, 3263–3279 (2018).
PACCOR to Start Bio-PP Products for Orkla (PACCOR, 27 September 2021); https://www.paccor.com/news-detail/paccor-to-start-bio-pp-products-for-orkla
Ma, K. D. et al. Open fermentative production of fuel ethanol from food waste by an acid-tolerant mutant strain of Zymomonas mobilis. Bioresour. Technol. 203, 295–302 (2016).
Limayem, A. & Ricke, S. C. Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog. Energy Combust. 38, 449–467 (2012).
Cui, X. K. et al. Robust enzymatic hydrolysis of Formiline-pretreated oil palm empty fruit bunches (EFB) for efficient conversion of polysaccharide to sugars and ethanol. Bioresour. Technol. 166, 584–591 (2014).
Sharma, S., Sharma, S., Singh, S., Nain, L. & Arora, A. Improving yeast strains for pentose hexose co-fermentation: successes and hurdles. In Proc. 1st International Conference on Recent Advances in Bionergy Research (eds Kumar, S. et al.) https://doi.org/10.1007/978-81-322-2773-1_3 (Springer, 2016).
Chacon-Navarrete, H., Martin, C. & Moreno-Garcia, J. Yeast immobilization systems for second-generation ethanol production: actual trends and future perspectives. Biofuel. Bioprod. Biorefin. 15, 1549–1565 (2021).
Cruz, M. L., de Resende, M. M. & Ribeiro, E. J. Improvement of ethanol production in fed-batch fermentation using a mixture of sugarcane juice and molasse under very high-gravity conditions. Bioprocress Biosyst. Eng. 44, 617–625 (2021).
Pandey, A. K. et al. Evaluation of divergent yeast genera for fermentation-associated stresses and identification of a robust sugarcane distillery waste isolate Saccharomyces cerevisiae NGY10 for lignocellulosic ethanol production in SHF and SSF. Biotechnol. Biofuels 12, 40 (2019).
Cunha, J. T. et al. Cell surface engineering of Saccharomyces cerevisiae for simultaneous valorization of corn cob and cheese whey via ethanol production. Energy Convers. Manag. https://doi.org/10.1016/j.enconman.2021.114359 (2021).
Yan, S. B., Chen, X. S., Wu, J. Y. & Wang, P. C. Ethanol production from concentrated food waste hydrolysates with yeast cells immobilized on corn stalk. Appl. Microbiol. Biotechnol. 94, 829–838 (2012).
Chen, Z., Huang, J. H., Wu, Y. & Liu, D. H. Metabolic engineering of Corynebacterium glutamicum for the de novo production of ethylene glycol from glucose. Metab. Eng. 33, 12–18 (2016).
Chae, T. U., Choi, S. Y., Ryu, J. Y. & Lee, S. Y. Production of ethylene glycol from xylose by metabolically engineered Escherichia coli. AIChE J. 64, 4193–4200 (2018).
Sandei, B., Massardier, V. & Brunel, R. Alternative building blocks sources for poly (ethylene terephthalate): a short review with socio-economical points of view. Front. Mater. 9, 1005770 (2022).
Maneffa, A., Priecel, P. & Lopez-Sanchez, J. A. Biomass-derived renewable aromatics: selective routes and outlook for p-xylene commercialisation. ChemSusChem 9, 2736–2748 (2016).
Baez, A., Cho, K. M. & Liao, J. C. High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl. Microbiol. Biotechnol. 90, 1681–1690 (2011).
Yamamoto, S., Suda, M., Niimi, S., Inui, M. & Yukawa, H. Strain optimization for efficient isobutanol production using Corynebacterium glutamicum under oxygen deprivation. Biotechnol. Bioeng. 110, 2938–2948 (2013).
Sarria, S., Wong, B., Martin, H. G., Keasling, J. D. & Peralta-Yahya, P. Microbial synthesis of pinene. ACS Synth. Biol. 3, 466–475 (2014).
Rolf, J., Julsing, M. K., Rosenthal, K. & Lutz, S. A gram-scale limonene production process with engineered Escherichia coli. Molecules 25, 1881 (2020).
Ling, C. et al. Muconic acid production from glucose and xylose in Pseudomonas putida via evolution and metabolic engineering. Nat. Commun. 13, 4925 (2022).
Becker, J., Kuhl, M., Kohlstedt, M., Starck, S. & Wittmann, C. Metabolic engineering of Corynebacterium glutamicum for the production of cis, cis-muconic acid from lignin. Microb. Cell Factories 17, 115 (2018).
Bramucci, M. G. et al. Pure bacterial isolates that convert p-xylene to terephthalic acid. Appl. Microbiol. Biotechnol. 58, 255–259 (2002).
Wang, J., Tian, J. & Xu, J. H. A method for producing terephthalic acid by Comamonas testosterone DSM6577. Chin. J. Catal. 27, 297–298 (2006).
Pressler, U. et al. Biotechnological perparation of alcohols, aldehydes and carboxylic acids. US patent US5753471A (1998).
Luo, Z. W. & Lee, S. Y. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli. Nat. Commun. 8, 15689 (2017).
Luo, Z. W., Choi, K. R. & Lee, S. Y. Improved terephthalic acid production from p-xylene using metabolically engineered Pseudomonas putida. Metab. Eng. 76, 75–86 (2023).
DeJong, E., Dam, R., Sipos, L., Den Ouden, D. & Gruter, G. J. Furandicarboxylic acid (FDCA) a versatile building block for a very interesting class of polyesters. Am. Chem. Soc. 241, 1–13 (2011).
Cui, Y. A., Deng, C., Fan, L. Q., Qiu, Y. J. & Zhao, L. M. Progress in the biosynthesis of bio-based PET and PEF polyester monomers. Green Chem. 25, 5836–5857 (2023).
Sheng, Y. Q., Tan, X., Zhou, X. & Xu, Y. Bioconversion of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) by a native obligate aerobic bacterium, Acinetobacter calcoaceticus NL14. Appl. Biochem. Biotech. 192, 455–465 (2020).
Koopman, F., Wierckx, N., de Winde, J. H. & Ruijssenaars, H. J. Efficient whole-cell biotransformation of 5-(hydroxymethyl)furfural into FDCA, 2,5-furandicarboxylic acid. Bioresour. Technol. 101, 6291–6296 (2010).
Yuan, H. B. et al. Enhanced 2,5-furandicarboxylic acid (FDCA) production in Raoultella ornithinolytica BF60 by manipulation of the key genes in FDCA biosynthesis pathway. J. Microbiol. Biotechnol. 28, 1999–2008 (2018).
Kurian, J. V. A new polymer platform for the future—Sorona® from corn derived 1,3-propanediol. J. Polym. Environ. 13, 159–167 (2005).
Wang, X. L. et al. Sequential fed-batch fermentation of 1,3-propanediol from glycerol by Clostridium butyricum DL07. Appl. Microbiol. Biotechnol. 104, 9179–9191 (2020).
Zhou, S. et al. Isolation and characterization of a Klebsiella pneumoniae strain from mangrove sediment for efficient biosynthesis of 1,3-propanediol. Sci. Bull. 60, 511–521 (2015).
Cervin, M. A., Soucaille, P. & Valle, F. Process for the biological production of 1,3-propanediol with high yield. US patent US7371558B2 (2008).
Zhou, S. F., Lama, S., Sankaranarayanan, M. & Park, S. Metabolic engineering of Pseudomonas denitrificans for the 1,3-propanediol production from glycerol. Bioresour. Technol. 292, 121933 (2019).
Gonzalez-Pajuelo, M. et al. Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol. Metab. Eng. 7, 329–336 (2005).
Kumar, R. et al. An overview of caprolactam synthesis. Catal. Rev. Sci. Eng. 61, 516–594 (2019).
Sarak, S. et al. One-pot biocatalytic synthesis of nylon monomers from cyclohexanol using Escherichia coli-based concurrent cascade consortia. Green Chem. 23, 9447–9453 (2021).
Deng, Y., Ma, L. Z. & Mao, Y. Biological production of adipic acid from renewable substrates: current and future methods. Biochem. Eng. J. 105, 16–26 (2016).
Chen, N., Wang, J. Y., Zhao, Y. Y. & Deng, Y. Metabolic engineering of Saccharomyces cerevisiae for efficient production of glucaric acid at high titer. Microb. Cell Factories 17, 67 (2018).
Chae, T. U., Ko, Y. S., Hwang, K. S. & Lee, S. Y. Metabolic engineering of Escherichia coli for the production of four-, five- and six-carbon lactams. Metab. Eng. 41, 82–91 (2017).
Zhao, M. et al. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway. Metab. Eng. 47, 254–262 (2018).
Picataggio, S. & Beardslee, T. Biological methods for preparing adpic acid. US patent US20120021474A1 (2012).
Fedorchuk, T. P. et al. One-pot biocatalytic transformation of adipic acid to 6-aminocaproic acid and 1,6-hexamethylenediamine using carboxylic acid reductases and transaminases. J. Am. Chem. Soc. 142, 1038–1048 (2020).
Wang, L., Li, G. H., Li, A. T. & Deng, Y. Directed synthesis of biobased 1,6-diaminohexane from adipicacid by rational regulation of a functional enzyme cascade in Escherichia coli. ACS Sustain. Chem. Eng. 11, 6011–6020 (2023).
Turk, S. C. H. J. et al. Metabolic engineering toward sustainable production of nylon-6. ACS Synth. Biol. 5, 65–73 (2016).
Han, T. & Lee, S. Y. Metabolic engineering of Corynebacterium glutamicum for the high-level production of valerolactam, a nylon-5 monomer. Metab. Eng. 79, 78–85 (2023).
Jeon, W. Y. et al. Microbial production of sebacic acid from a renewable source: production, purification, and polymerization. Green Chem. 21, 6491–6501 (2019).
Clomburg, J. M. et al. Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metab. Eng. 28, 202–212 (2015).
Bioplastic Materials (European Bioplastics e.V., 2022); https://www.european-bioplastics.org/bioplastics/materials/
Aversa, C., Barletta, M., Cappiello, G. & Gisario, A. Compatibilization strategies and analysis of morphological features of poly (butylene adipate-co-terephthalate) (PBAT)/poly(lactic acid) PLA blends: a state-of-art review. Eur. Polym. J. 173, 111304 (2022).
Yim, H. et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 7, 445–452 (2011).
Burgard, A., Burk, M. J., Osterhout, R., Van Dien, S. & Yim, H. Development of a commercial scale process for production of 1,4-butanediol from sugar. Curr. Opin. Biotechnol. 42, 118–125 (2016).
Jansen, M. L. A. & van Gulik, W. M. Towards large scale fermentative production of succinic acid. Curr. Opin. Biotechnol. 30, 190–197 (2014).
Lee, S. J., Song, H. & Lee, S. Y. Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl. Environ. Microbiol. 72, 1939–1948 (2006).
Li, C., Gao, S., Yang, X. F. & Lin, C. S. K. Green and sustainable succinic acid production from crude glycerol by engineered Yarrowia lipolytica via agricultural residue based in situ fibrous bed bioreactor. Bioresour. Technol. 249, 612–619 (2018).
Chung, S. C., Park, J. S., Yun, J. & Park, J. H. Improvement of succinate production by release of end-product inhibition in Corynebacterium glutamicum. Metab. Eng. 40, 157–164 (2017).
Ahn, J. H., Lee, J. A., Bang, J. & Lee, S. Y. Membrane engineering via trans-unsaturated fatty acids production improves succinic acid production in Mannheimia succiniciproducens. J. Ind. Microbiol. Biotechnol. 45, 555–566 (2018).
Ahn, J. H. et al. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase. Nat. Commun. 11, 1970 (2020).
de Oliveira, P. Z., de Souza Vandenberghe, L. P., de Mello, A. F. M. & Soccol, C. R. A concise update on major poly-lactic acid bioprocessing barriers. Bioresour. Technol. Rep. 18, 101094 (2022).
Moon, S. K., Wee, Y. J. & Choi, G. W. A novel lactic acid bacterium for the production of high purity l-lactic acid, Lactobacillus paracasei subsp. paracasei CHB2121. J. Biosci. Bioeng. 114, 155–159 (2012).
Romani, A., Yanez, R., Garrote, G. & Alonso, J. L. SSF production of lactic acid from cellulosic biosludges. Bioresour. Technol. 99, 4247–4254 (2008).
Ou, M. S., Ingram, L. O. & Shanmugam, K. T. l(+)-Lactic acid production from non-food carbohydrates by thermotolerant Bacillus coagulans. J. Ind. Microbiol. Biotechnol. 38, 599–605 (2011).
Hidese, R., Matsuda, M., Osanai, T., Hasunuma, T. & Kondo, A. Malic enzyme facilitates d-lactate production through increased pyruvate supply during anoxic dark fermentation in Synechocystis sp. PCC 6803. ACS Synth. Biol. 9, 260–268 (2020).
Wang, X. D., Cui, Z. Z., Sun, X., Wang, Z. W. & Chen, T. Production of 3-hydroxypropionic acid from renewable substrates by metabolically engineered microorganisms: a review. Molecules 28, 1888 (2023).
LETZero Product Book, 16–17 (LG Chem, 2021).
Kim, J. W., Ko, Y. S., Chae, T. U. & Lee, S. Y. High-level production of 3-hydroxypropionic acid from glycerol as a sole carbon source using metabolically engineered Escherichia coli. Biotechnol. Bioeng. 117, 2139–2152 (2020).
Chen, Z. et al. Metabolic engineering of Corynebacterium glutamicum for the production of 3-hydroxypropionic acid from glucose and xylose. Metab. Eng. 39, 151–158 (2017).
Zhao, P., Ma, C. L., Xu, L. D. & Tian, P. F. Exploiting tandem repetitive promoters for high-level production of 3-hydroxypropionic acid. Appl. Microbiol. Biotechnol. 103, 4017–4031 (2019).
Chu, H. S. et al. Direct fermentation route for the production of acrylic acid. Metab. Eng. 32, 23–29 (2015).
Ko, Y. S., Kim, J. W., Chae, T. U., Song, C. W. & Lee, S. Y. A novel biosynthetic pathway for the production of acrylic acid through β-alanine route in Escherichia coli. ACS Synth. Biol. 9, 1150–1159 (2020).
Chae, T. U., Kim, W. J., Choi, S., Park, S. J. & Lee, S. Y. Metabolic engineering of Escherichia coli for the production of 1,3-diaminopropane, a three carbon diamine. Sci. Rep. 5, 13040 (2015).
Noh, M., Yoo, S. M., Kim, W. J. & Lee, S. Y. Gene expression knockdown by modulating synthetic small RNA expression in Escherichia coli. Cell Syst. 5, 418–426 (2017).
Wang, J. et al. A novel process for cadaverine bio-production using a consortium of two engineered Escherichia coli. Front. Microbiol. https://doi.org/10.3389/fmicb.2018.01312 (2018).
Song, C. W., Kim, J. W., Cho, I. J. & Lee, S. Y. Metabolic engineering of Escherichia coli for the production of 3-hydroxypropionic acid and malonic acid through β-alanine route. ACS Synth. Biol. 5, 1256–1263 (2016).
Lee, J. A., Ahn, J. H., Kim, I., Li, S. & Lee, S. Y. Synthesis, characterization, and application of fully bio-based and biodegradable nylon-4,4 and -5,4. ACS Sustain. Chem. Eng. 8, 5604–5614 (2020).
Kind, S. et al. From zero to hero—production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab. Eng. 25, 113–123 (2014).
Kim, H. T. et al. Metabolic engineering of Corynebacterium glutamicum for the high-level production of cadaverine that can be used for the synthesis of biopolyamide 510. ACS Sustain. Chem. Eng. 6, 5296–5305 (2018).
Han, T., Kim, G. B. & Lee, S. Y. Glutaric acid production by systems metabolic engineering of an l-lysine-overproducing Corynebacterium glutamicum. Proc. Natl Acad. Sci. USA 117, 30328–30334 (2020).
Michinobu, T. et al. Polyesters of 2-pyrone-4,6-dicarboxylic acid (PDC) obtained from a metabolic intermediate of lignin. Polym. J. 40, 68–75 (2008).
Otsuka, Y. et al. Efficient production of 2-pyrone 4,6-dicarboxylic acid as a novel polymer-based material from protocatechuate by microbial function. Appl. Microbiol. Biotechnol. 71, 608–614 (2006).
Luo, Z. W., Kim, W. J. & Lee, S. Y. Metabolic engineering of Escherichia coli for efficient production of 2-pyrone-4,6-dicarboxylic acid from glucose. ACS Synth. Biol. 7, 2296–2307 (2018).
Zhou, D. et al. Multi-step biosynthesis of the biodegradable polyester monomer 2-pyrone-4,6-dicarboxylic acid from glucose. Biotechnol. Biofuels Bioprod. https://doi.org/10.1186/s13068-023-02350-y (2023).
Liu, T. Q. et al. Synthesis of polymandelide: a degradable polylactide derivative with polystyrene-like properties. Macromolecules 40, 6040–6047 (2007).
Sun, Z. T. et al. Metabolic engineering of the l-phenylalanine pathway in Escherichia coli for the production of S- or R-mandelic acid. Microb. Cell Factories 10, 71 (2011).
Choi, S. Y. et al. Microbial polyhydroxyalkanoates and nonnatural polyesters. Adv. Mater. 32, e1907138 (2020).
Sudesh, K., Abe, H. & Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog. Polym. Sci. 25, 1503–1555 (2000).
Markets and Markets. Polyhydroxyalkanoate (PHA) Market by Type (Short Chain Length, Medium Chain Length), Production Methods (Sugar Fermentation, Vegetable Oil Fermentation), Application (Packaging & Food Services, Biomedical), and Region—Global Forecast to 2028 https://www.marketsandmarkets.com/Market-Reports/pha-market-395.html (2023).
Jung, Y. K., Kim, T. Y., Park, S. J. & Lee, S. Y. Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol. Bioeng. 105, 161–171 (2010).
Choi, S. Y. et al. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat. Biotechnol. 34, 435–440 (2016).
Ryu, H. W., Hahn, S. K., Chang, Y. K. & Chang, H. N. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phospate limitation. Biotechnol. Bioeng. 55, 28–32 (1997).
Wang, F. & Lee, S. Y. Poly(3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 63, 3703–3706 (1997).
Suriyamongkol, P., Weselake, R., Narine, S., Moloney, M. & Shah, S. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants—a review. Biotechnol. Adv. 25, 148–175 (2007).
Choi, J. I., Lee, S. Y. & Han, K. Cloning of the Alcaligenes latus polyhydroxyalkanoate biosynthesis genes and use of these genes for enhanced production of poly(3-hydroxybutyrate) in Escherichia coli. Appl. Environ. Microbiol. 64, 4897–4903 (1998).
Choi, J. & Lee, S. Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol. Bioeng. 62, 546–553 (1999).
Lee, S. Y. & Choi, J. I. High level production of supra molecular weight poly(3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnol. Bioprocess Eng. 9, 196–200 (2004).
Doi, Y., Segawa, A. & Kunioka, M. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int. J. Biol. Macromol. 12, 106–111 (1990).
Andreessen, B., Taylor, N. & Steinbuchela, A. Poly(3-hydroxypropionate): a promising alternative to fossil fuel-based materials. Appl. Environ. Microbiol. 80, 6574–6582 (2014).
Kim, B. S. et al. Production of poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) by fed-batch culture of Alcaligenes eutrophus with substrate control using online glucose analyzer. Enzyme Micro. Technol. 16, 556–561 (1994).
Aldor, A. S., Kim, S. W., Prather, K. L. J. & Keasling, J. D. Metabolic engineering of a novel propionate-independent pathway for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 68, 3848–3854 (2002).
Li, Z. J. et al. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab. Eng. 12, 352–359 (2010).
Fukui, T., Suzuki, M., Tsuge, T. & Nakamura, S. Microbial synthesis of poly((R)-3-hydroxybutyrate-co-3-hydroxypropionate) from unrelated carbon sources by engineered Cupriavidus necator. Biomacromolecules 10, 700–706 (2009).
Wang, Q. et al. Biosynthesis of poly(3-hydroxypropionate-co-3-hydroxybutyrate) with fully controllable structures from glycerol. Bioresour. Technol. 142, 741–744 (2013).
Noda, I., Lindsey, S. B. & Caraway, D. in Plastics from Bacteria. Microbiology Monographs Vol. 14 (ed. Chen, G. Q.) 237–255 (2010).
Lee, S. H. et al. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol. Bioeng. 67, 240–244 (2000).
Kahar, P., Tsuge, T., Taguchi, K. & Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stabil. 83, 79–86 (2004).
Riedel, S. L. et al. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol. Bioeng. 109, 74–83 (2012).
Zhang, M., Kurita, S., Orita, I., Nakamura, S. & Fukui, T. Modification of acetoacetyl-CoA reduction step in Ralstonia eutropha for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from structurally unrelated compounds. Microb. Cell Factories 18, 147 (2019).
Utsunomia, C., Ren, Q. & Zinn, M. Poly(4-hydroxybutyrate): current state and perspectives. Front. Bioeng. Biotechnol. 8, 257 (2020).
Zhou, X. Y. et al. Hyperproduction of poly(4-hydroxybutyrate) from glucose by recombinant Escherichia coli. Micro. Cell Factories 11, 54 (2012).
Huisman, G. W., Skraly, F., Martin, D. P. & Peoples, O. P. Biological systems for manufacture of polyhydroxyalkanoate polymers containing 4-hydroxyacids. US patent US6316262B1 (2001).
Choi, S. Y. et al. Metabolic engineering for the synthesis of polyesters: a 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters. Metab. Eng. 58, 47–81 (2020).
Sohn, Y. J., Son, J., Lim, H. J., Lim, S. H. & Park, S. J. Valorization of lignocellulosic biomass for polyhydroxyalkanoate production: status and perspectives. Bioresour. Technol. 360, 127575 (2022).
Nielsen, C., Rahman, A., Rehman, A. U., Walsh, M. K. & Miller, C. D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 10, 1338–1352 (2017).
Yoon, J. & Oh, M. K. Strategies for biosynthesis of C1 gas-derived polyhydroxyalkanoates: a review. Bioresour. Technol. 344, 126307 (2022).
Levett, I. et al. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—the case for thermophilic bioprocessing. J. Environ. Chem. Eng. https://doi.org/10.1016/j.jece.2016.07.033 (2016).
Kim, G. B., Choi, S. Y., Cho, I. J., Ahn, D. & Lee, S. Y. Metabolic engineering for sustainability and health. Trends Biotechnol. 41, 425–451 (2023).
Tang, R., Yuan, X. & Yang, J. Problems and corresponding strategies for converting CO2 into value-added products in Cupriavidus necator H16 cell factories. Biotechnol. Adv. 67, 108183 (2023).
Poblete-Castro, I., Rodriguez, A. L., Lam, C. M. & Kessler, W. Improved production of medium-chain-length polyhydroxyalkanoates in glucose-based fed-batch cultivations of metabolically engineered Pseudomonas putida strains. J. Microbiol. Biotechnol. 24, 59–69 (2014).
Chen, G. Q. & Jiang, X. R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 50, 94–100 (2018).
Chen, X. B. et al. Engineering Halomonas bluephagenesis TD01 for non-sterile production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Bioresour. Technol. 244, 534–541 (2017).
Zheng, Y. Y., Yuan, Q. Q., Yang, X. Y. & Ma, H. W. Engineering Escherichia coli for poly-(3-hydroxybutyrate) production guided by genome-scale metabolic network analysis. Enzyme Microb. Technol. 106, 60–66 (2017).
Lin, Z. Q. et al. Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Micro. Cell Factories 14, 185 (2015).
Valentin, H. E. & Steinbuchel, A. Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl. Microbiol. Biotechnol. 40, 699–709 (1994).
Taguchi, S. et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl Acad. Sci. USA 105, 17323–17327 (2008).
Jung, Y. K. & Lee, S. Y. Efficient production of polylactic acid and its copolymers by metabolically engineered Escherichia coli. J. Biotechnol. 151, 94–101 (2011).
Choi, S. Y. et al. Engineering the xylose-catabolizing Dahms pathway for production of poly(d-lactate-co-glycolate) and poly(d-lactate-co-glycolate-co-D-2-hydroxybutyrate) in Escherichia coli. Microb. Biotechnol. 10, 1353–1364 (2017).
Yang, J. E. et al. One-step fermentative production of aromatic polyesters from glucose by metabolically engineered Escherichia coli strains. Nat. Commun. 9, 79 (2018).
Pathak, V. M. Review on the current status of polymer degradation: a microbial approach. Bioresour. Bioprocess. 4, 135 (2017).
Filiciotto, L. & Rothenberg, G. Biodegradable plastics: standards, policies, and impacts. ChemSusChem 14, 56–72 (2021).
Kourmentza, C. et al. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 4, 55 (2017).
Meereboer, K. W., Misra, M. & Mohanty, A. K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 22, 5519–5558 (2020).
Volova, T. G., Prudnikova, S. V., Vinogradova, O. N., Syrvacheva, D. A. & Shishatskaya, E. I. Microbial degradation of polyhydroxyalkanoates with different chemical compositions and their biodegradability. Microb. Ecol. 73, 353–367 (2017).
Papaneophytou, C. P., Pantazaki, A. A. & Kyriakidis, D. A. An extracellular polyhydroxybutyrate depolymerase in Thermus thermophilus HB8. Appl. Microbiol. Biotechnol. 83, 659–668 (2009).
Knoll, M., Hamm, T. M., Wagner, F., Martinez, V. & Pleiss, J. The PHA depolymerase engineering database: a systematic analysis tool for the diverse family of polyhydroxyalkanoate (PHA) depolymerases. BMC Bioinform. 10, 89 (2009).
Hiraishi, T., Komiya, N. & Maeda, M. Y443F mutation in the substrate-binding domain of extracellular PHB depolymerase enhances its PHB adsorption and disruption abilities. Polym. Degrad. Stabil. 95, 1370–1374 (2010).
Delacuvellerie, A. et al. Microbial biofilm composition and polymer degradation of compostable and non-compostable plastics immersed in the marine environment. J. Hazard. Mater. 419, 126526 (2021).
Narancic, T. et al. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 52, 10441–10452 (2018).
Samantaray, P. K., Little, A., Wemyss, A. M., Iacovidou, E. & Wan, C. Y. Design and control of compostability in synthetic biopolyesters. ACS Sustain. Chem. Eng. 9, 9151–9164 (2021).
Zaaba, N. F. & Jaafar, M. A review on degradation mechanisms of polylactic acid: hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 60, 2061–2075 (2020).
Tournier, V. et al. Enzymes? Power for plastics degradation. Chem. Rev. 123, 5612–5701 (2023).
Williams, D. F. Enzymic hydrolysis of polylactic acid. Eng. Med. 10, 5–7 (1981).
Kawai, F. et al. Different enantioselectivity of two types of poly(lactic acid) depolymerases toward poly(l-lactic acid) and poly(d-lactic acid). Polym. Degrad. Stabil. 96, 1342–1348 (2011).
Ribitsch, D. et al. Small cause, large effect: structural characterization of cutinases from Thermobifida cellulosilytica. Biotechnol. Bioeng. 114, 2481–2488 (2017).
Marty, A., Duquesne, S., Guicherd, M., Gueroult, M. & André, I. Novel proteases and uses thereof. US patent US20200385698A1 (2020).
Youngpreda, A. et al. Optimization of poly(dl-lactic acid) degradation and evaluation of biological re-polymerization. J. Polym. Environ. 25, 1131–1139 (2017).
Lomthong, T. et al. Poly(l-lactide)-degrading enzyme from Laceyella sacchari LP175: cloning, sequencing, expression, characterization and its hydrolysis of poly(L-lactide) polymer. Chiang Mai J. Sci. 46, 417–430 (2019).
Kleeberg, I., Hetz, C., Kroppenstedt, R. M., Muller, R. J. & Deckwer, W. D. Biodegradation of aliphatic-aromatic copolyesters by Thermomonospora fusca and other thermophilic compost isolates. Appl. Environ. Microbiol. 64, 1731–1735 (1998).
Witt, U., Muller, R. J. & Deckwer, W. D. New biodegradable polyester-copolymers from commodity chemicals with favorable use properties. J. Environ. Polym. Degrad. 3, 215–223 (1995).
Jia, H. et al. Degradation of poly(butylene adipate-co-terephthalate) by Stenotrophomonas sp. YCJ1 isolated from farmland soil. J. Environ. Sci. 103, 50–58 (2021).
Tesei, D. et al. Shotgun proteomics reveals putative polyesterases in the secretome of the rock-inhabiting fungus Knufia chersonesos. Sci. Rep. 10, 9770 (2020).
Biundo, A. et al. Characterization of a poly(butylene adipate-co-terephthalate)-hydrolyzing lipase from Pelosinus fermentans. Appl. Microbiol. Biotechnol. 100, 1753–1764 (2016).
Kanwal, A., Zhang, M., Sharaf, F. & Li, C. T. Enzymatic degradation of poly (butylene adipate co-terephthalate) (PBAT) copolymer using lipase B from Candida antarctica (CALB) and effect of PBAT on plant growth. Polym. Bull. 79, 9059–9073 (2022).
Kawai, F., Kawabata, T. & Oda, M. Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustain. Chem. Eng. 8, 8894–8908 (2020).
Yang, Y. et al. Complete bio-degradation of poly(butylene adipate-co-terephthalate) via engineered cutinases. Nat. Commun. 14, 1645 (2023).
Fields, R. D., Rodriguez, F. & Finn, R. K. Microbial degradation of polyesters: polycaprolactone degraded by P. pullulans. J. Appl. Polym. Sci. 18, 3571–3579 (1974).
Darby, R. T. & Kaplan, A. M. Fungal susceptibility of polyurethanes. Appl. Microbiol. Biotechnol. 16, 900–905 (1968).
Zhang, Y., Pedersen, J. N., Eser, B. E. & Guo, Z. Biodegradation of polyethylene and polystyrene: from microbial deterioration to enzyme discovery. Biotechnol. Adv. 60, 107991 (2022).
Kawai, F., Kawabata, T. & Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 103, 4253–4268 (2019).
Sulaiman, S. et al. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 78, 1556–1562 (2012).
Ronkvist, A. M., Xie, W. C., Lu, W. H. & Gross, R. A. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 42, 5128–5138 (2009).
Yoshida, S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199 (2016).
Chow, J. et al. The first archaeal PET-degrading enzyme belongs to the feruloyl-esterase family. Preprint at bioRxiv https://doi.org/10.1101/2022.10.14.512230 (2022).
Joo, S. et al. Structural insight into molecular mechanism of poly (ethylene terephthalate) degradation. Nat. Commun. 9, 382 (2018).
Son, H. F. et al. Rational protein engineering of thermo-stable PETase from Ideonella sakaiensis for highly efficient PET degradation. ACS Catal. 9, 3519–3526 (2019).
Bell, E. L. et al. Directed evolution of an efficient and thermostable PET depolymerase. Nat. Catal. 5, 673–681 (2022).
Lu, H. Y. et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604, 662–667 (2022).
Tournier, V. et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 580, 216–219 (2020).
White, M. F. M. & Wallace, S. A new PETase from the human saliva metagenome and its functional modification via genetic code expansion in bacteria. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202216963 (2023).
Chen, Z. Z. et al. Efficient biodegradation of highly crystallized polyethylene terephthalate through cell surface display of bacterial PETase. Sci. Total Environ. 709, 136138 (2020).
Howard, S. A. & McCarthy, R. R. Modulating biofilm can potentiate activity of novel plastic-degrading enzymes. npj Biofilms Microbiomes 9, 72 (2023).
Restrepo-Florez, J. M., Bassi, A. & Thompson, M. R. Microbial degradation and deterioration of polyethylene—a review. Int. Biodeterior. Biodegradation 88, 83–90 (2014).
Ren, L. et al. Biodegradation of polyethylene by Enterobacter sp. D1 from the guts of wax moth Galleria mellonella. Catal. Rev. 16, 1941 (2019).
Mukherjee, S. & Kundu, P. P. Alkaline fungal degradation of oxidized polyethylene in black liquor: studies on the effect of lignin peroxidases and manganese peroxidases. J. Appl. Polym. Sci. https://doi.org/10.1002/app.40738 (2014).
Santo, M., Weitsman, R. & Sivan, A. The role of the copper-binding enzyme—laccase—in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegradation 84, 204–210 (2013).
Yao, C., Xia, W., Dou, M., Du, Y. & Wu, J. Oxidative degradation of UV-irradiated polyethylene by laccase-mediator system. J. Hazard. Mater. 440, 129709 (2022).
Zampolli, J. et al. Oxidative degradation of polyethylene by two novel laccase-like multicopper oxidases from Rhodococcus opacus R7. Environ. Technol. Innov. 32, 103273 (2023).
Jeon, H. J. & Kim, M. N. Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. Int. Biodeterior. Biodegradation 103, 141–146 (2015).
Sadler, J. C. & Wallace, S. Microbial synthesis of vanillin from waste poly(ethylene terephthalate). Green Chem. 23, 4665–4672 (2021).
Kim, H. T. et al. Biological valorization of poly(ethylene terephthalate) monomers for upcycling waste PET. ACS Sustain. Chem. Eng. 7, 19396–19406 (2019).
Kenny, S. T. et al. Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 95, 623–633 (2012).
Werner, A. Z. et al. Tandem chemical deconstruction and biological upcycling of poly(ethylene terephthalate) to β-ketoadipic acid by Pseudomonas putida KT2440. Metab. Eng. 67, 250–261 (2021).
Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science 378, 207–211 (2022).
Spierling, S. et al. Bio-based plastics—a review of environmental, social and economic impact assessments. J. Clean. Prod. 185, 476–491 (2018).
Nicholas Institute for Energy, Environment & Sustainability Plastics Policy Inventory Search (Duke University); https://nicholasinstitute.duke.edu/plastics-policy-inventory/search
Dutta, K., Daverey, A. & Lin, J. G. Evolution retrospective for alternative fuels: first to fourth generation. Renew. Energy 69, 114–122 (2014).
Acknowledgements
This work is supported by the Development of Platform Technologies of Microbial Cell Factories for the Next-Generation Biorefineries Project (2022M3J5A1056117) and the Development of Platform Technology for the Production of Novel Aromatic Bioplastic Using Microbial Cell Factories Project (2022M3J4A1053699) from the National Research Foundation supported by the Korean Ministry of Science and ICT.
Author information
Authors and Affiliations
Contributions
S.Y.L. conceived the project. S.Y.C., I.J.C., Y.L., H.E.Y., M.K. and S.Y.L. wrote the manuscript. S.Y.C., I.J.C., Y.L., H.E.Y. and M.K. prepared the figures and table.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Kechun Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, S.Y., Lee, Y., Yu, H.E. et al. Sustainable production and degradation of plastics using microbes. Nat Microbiol 8, 2253–2276 (2023). https://doi.org/10.1038/s41564-023-01529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-023-01529-1