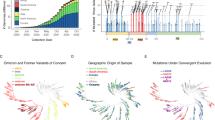
Abstract
Since SARS-CoV-2 BA.5 (Omicron) emerged and spread in 2022, Omicron lineages have markedly diversified. Here we review the evolutionary trajectories and processes that underpin the emergence of these lineages, and identify the most prevalent sublineages. We discuss the potential origins of second-generation BA.2 lineages. Simple and complex recombination, antigenic drift and convergent evolution have enabled SARS-CoV-2 to accumulate mutations that alter its antigenicity. We also discuss the potential evolutionary trajectories of SARS-CoV-2 in the future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carabelli, A. M. et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat. Rev. Microbiol. 21, 162–177 (2023).
Harvey, W. T. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19, 409–424 (2021).
Markov, P. V. et al. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 21, 361–379 (2023).
Schmidt, F. et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature 600, 512–516 (2021).
Greaney, A. J. et al. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe 29, 463–476 (2021).
Martin, D. P. et al. The emergence and ongoing convergent evolution of the SARS-CoV-2 N501Y lineages. Cell 184, 5189–5200 (2021).
Cao, Y. et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 614, 521–529 (2023).
Starr, T. N. et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell 182, 1295–1310 (2020).
Zahradník, J. et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat. Microbiol. 6, 1188–1198 (2021).
Viana, R. et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 603, 679–686 (2022).
Tegally, H. et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 592, 438–443 (2021).
Faria, N. R. et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 372, 815–821 (2021).
Rambaut, A. et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. virological.org https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563 (2020).
Hill, V. et al. The origins and molecular evolution of SARS-CoV-2 lineage B.1.1.7 in the UK. Virus Evol. 8, veac080 (2022).
Ruis, C. et al. Preadaptation of pandemic GII.4 noroviruses in unsampled virus reservoirs years before emergence. Virus Evol. 6, veaa067 (2020).
Kemp, S. A. et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 592, 277–282 (2021).
Wilkinson, S. A. J. et al. Recurrent SARS-CoV-2 mutations in immunodeficient patients. Virus Evol. 8, veac050 (2022).
Harari, S. et al. Drivers of adaptive evolution during chronic SARS-CoV-2 infections. Nat. Med. 28, 1501–1508 (2022).
Mallapaty, S. Where did Omicron come from? Three key theories. Nature 602, 26–28 (2022).
Tegally, H. et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 28, 1785–1790 (2022).
Karyakarte, R. P. et al. An early and preliminary assessment of the clinical severity of the emerging SARS-CoV-2 Omicron variants in Maharashtra, India. Cureus 14, e31352 (2022).
Regarding Mutant Strains of the New Coronavirus (SARS-CoV-2), which are Concerned about Increased Infection and Transmissibility and Changes in Antigenicity (20th Report) (Japanese National Institute of infectious Diseases (NIID), 2022); https://www.niid.go.jp/niid/ja/2019-ncov/2551-cepr/11469-sars-cov-2-20.html
Hisner, R. 2nd-Generation BA.2 saltation lineage, >30 spike mutations (3 seq, 2 countries, Aug 14) #2183. GitHub https://github.com/cov-lineages/pango-designation/issues/2183 (2023).
Dijokaite-Guraliuc, A. et al. Rapid escape of new SARS-CoV-2 Omicron variants from BA.2-directed antibody responses. Cell Rep. 42, 112271 (2023).
SARS-CoV-2 Variants of Concern and Variants under Investigation in England Technical Briefing 49 (UK Health Security Agency, 2023).
Jackson, B. et al. Generation and transmission of interlineage recombinants in the SARS-CoV-2 pandemic. Cell 184, 5179–5188 (2021).
Gutierrez, B. et al. Emergence and widespread circulation of a recombinant SARS-CoV-2 lineage in North America. Cell Host Microbe 30, 1112–1123 (2022).
Simon-Loriere, E. et al. Rapid characterization of a Delta-Omicron SARS-CoV-2 recombinant detected in Europe. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-1502293/v1 (2022).
Pybus, O. Pango lineage Nomenclature: provisional rules for naming recombinant lineages. virological.org https://virological.org/t/pango-lineage-nomenclature-provisional-rules-for-naming-recombinant-lineages/657 (2021).
Sheward, D. J. et al. Omicron sublineage BA.2.75.2 exhibits extensive escape from neutralising antibodies. Lancet Infect. Dis. 22, 1538–1540 (2022).
Akerman, A. et al. Emergence and antibody evasion of BQ, BA.2.75 and SARS-CoV-2 recombinant sub-lineages in the face of maturing antibody breadth at the population level. eBioMedicine 90, 104545 (2023).
Silcn. XBB.1*/BA.2.75*/XBB.1* recombinant with S:F486P (8 seq, Malaysia) #1532. GitHub https://github.com/cov-lineages/pango-designation/issues/1532 (2023).
SARS-CoV 2 Sequencing Update 15 July 2022 (Network for Genomic Surveillance in South Africa, 2022).
Pangilinan, E. A. R. et al. Analysis of SARS-CoV-2 recombinant lineages XBC and XBC.1 in the Philippines and evidence for Delta-Omicron co-infection as a potential origin. Preprint at bioRxiv https://doi.org/10.1101/2023.04.12.534029 (2023).
Chaguza, C. et al. Accelerated SARS-CoV-2 intrahost evolution leading to distinct genotypes during chronic infection. Preprint at medRxiv https://doi.org/10.1101/2022.06.29.22276868 (2022).
Smith, D. J. et al. Mapping the antigenic and genetic evolution of influenza virus. Science 305, 371–376 (2004).
Eguia, R. T. et al. A human coronavirus evolves antigenically to escape antibody immunity. PLoS Pathog. 17, e1009453 (2021).
Moulana, A. et al. Compensatory epistasis maintains ACE2 affinity in SARS-CoV-2 Omicron BA.1. Nat. Commun. 13, 7011 (2022).
Yue, C. et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 23, 278–280 (2023).
Focosi, D., Quiroga, R., McConnell, S., Johnson, M. C. & Casadevall, A. Convergent evolution in SARS-CoV-2 spike creates a variant soup from which new COVID-19 waves emerge. Int. J. Mol. Sci. 24, 2264 (2023).
Starr, T. N. et al. Deep mutational scans for ACE2 binding, RBD expression, and antibody escape in the SARS-CoV-2 Omicron BA.1 and BA.2 receptor-binding domains. PLoS Pathog. 18, e1010951 (2022).
Cox, M. et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat. Rev. Microbiol. 21, 112–124 (2023).
McCallum, M. et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 184, 2332–2347 (2021).
Gerdol, M., Dishnica, K. & Giorgetti, A. Emergence of a recurrent insertion in the N-terminal domain of the SARS-CoV-2 spike glycoprotein. Virus Res. 310, 198674 (2022).
Greco, S. & Gerdol, M. Independent acquisition of short insertions at the RIR1 site in the spike N-terminal domain of the SARS-CoV-2 BA.2 lineage. Transbound. Emerg. Dis. 69, e3408–e3415 (2022).
Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602, 657–663 (2022).
Yisimayi, A. et al. Repeated Omicron infection alleviates SARS-CoV-2 immune imprinting. Preprint at bioRxiv https://doi.org/10.1101/2023.05.01.538516 (2023).
Koelle, K., Cobey, S., Grenfell, B. & Pascual, M. Epochal evolution shapes the phylodynamics of interpandemic influenza A (H3N2) in humans. Science 314, 1898–1903 (2006).
Barnes, C. O. et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687 (2020).
Wang, Y. et al. A large-scale systematic survey reveals recurring molecular features of public antibody responses to SARS-CoV-2. Immunity 55, 1105–1117 (2022).
Twohig, K. A. et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect. Dis. 22, 35–42 (2022).
Harari, S., Miller, D., Fleishon, S., Burstein, D. & Stern, A. Using big sequencing data to identify chronic SARS-coronavirus-2 infections. Preprint at bioRxiv https://doi.org/10.1101/2023.07.16.549184 (2023).
Domańska-Blicharz, K. et al. Cryptic SARS-CoV-2 lineage identified on two mink farms as a possible result of long-term undetected circulation in an unknown animal reservoir, Poland, November 2022 to January 2023. Eurosurveillance 28, 2300188 (2023).
Pickering, B. et al. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nat. Microbiol. 7, 2011–2024 (2022).
Brito, A. F. et al. Global disparities in SARS-CoV-2 genomic surveillance. Nat. Commun. 13, 7003 (2022).
Shu, Y. & McCauley, J. GISAID: global initiative on sharing all influenza data—from vision to reality. Eurosurveillance 22, 30494 (2017).
Murrell, B. SARS-CoV-2 lineage competition. GitHub https://github.com/MurrellGroup/lineages (2023).
Lacek, K. A. et al. SARS-CoV-2 Delta-Omicron recombinant viruses, United States. Emerg. Infect. Dis. 28, 1442–1445 (2022).
Acknowledgements
We gratefully acknowledge all data contributors—the authors, their originating laboratories that were responsible for obtaining the specimens, and their submitting laboratories for generating the genetic sequences and metadata and sharing through the GISAID initiative56, on which this research is based. The growth competition analysis from https://github.com/MurrellGroup/lineages (ref. 57) was performed on all available GISAID sequences, downloaded as a package on 22 May 2023. We thank the Pango lineage designation committee and wider contributors for identifying and naming lineages; the teams behind Pangolin, Nextstrain, covSPECTRUM and UShER (particularly A. Hinrichs) for developing tools that are used to identify and survey lineages; and S. Fleishon for his valuable feedback and discussions. T.P.P. is funded by the MRC-funded G2P-UK National Virology Consortium (MR/W005611/1); O.G.P. and C. Ruis are supported by the Oxford Martin School; O.G.P. acknowledges assistance from S. Attwood, funded by the Horizon Europe Research and Innovation programme under grant agreement no. 871075 (ELIXIR-CONVERGE); and D.J.S., K.S. and B.M. were supported by funding from SciLifeLab’s Pandemic Laboratory Preparedness programme (VC-2022-0028 and VC-2021-0033) and from the Erling Persson Foundation (ID: 2021 0125).
Author information
Authors and Affiliations
Contributions
C. Roemer, D.J.S., R.H., F.G., H.S., N.F., J.S., B.M. and T.P.P. researched data. C. Roemer, D.J.S., R.H., F.G., H.S., N.F., J.S., A.O., A.R., O.G.P. and C. Ruis substantially contributed to discussion of content. K.S., B.M. and T.P.P. performed updated analyses. D.J.S. and T.P.P. wrote the first draft. All authors reviewed and edited the paper before submission.
Corresponding author
Ethics declarations
Competing interests
A.O., A.R. and O.G.P. have undertaken consulting for AstraZeneca AB relating to the genetic diversity and classification of SARS-COV-2 lineages. D.J.S. also consults for AstraZeneca AB on matters related to monoclonal antibody therapeutics for COVID-19. At the time of final submission of this manuscript, F.G. was a contractor for Invivyd, a company that develops monoclonal antibodies to treat COVID-19. The other authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Rowena Bull, Frank Kirchhoff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roemer, C., Sheward, D.J., Hisner, R. et al. SARS-CoV-2 evolution in the Omicron era. Nat Microbiol 8, 1952–1959 (2023). https://doi.org/10.1038/s41564-023-01504-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-023-01504-w