Abstract
Effective mRNA SARS-CoV-2 vaccines are available but need to be stored in freezers, limiting their use to countries that have appropriate storage capacity. ChulaCov19 is a prefusion non-stabilized SARS-CoV-2 spike-protein-encoding, nucleoside-modified mRNA, lipid nanoparticle encapsulated vaccine that we report to be stable when stored at 2–8 °C for up to 3 months. Here we report safety and immunogenicity data from a phase I open-label, dose escalation, first-in-human trial of the ChulaCov19 vaccine (NCT04566276). Seventy-two eligible volunteers, 36 of whom were aged 18–55 (adults) and 36 aged 56–75 (elderly), were enroled. Two doses of vaccine were administered 21 d apart at 10, 25 or 50 μg per dose (12 per group). The primary outcome was safety and the secondary outcome was immunogenicity. All three dosages of ChulaCov19 were well tolerated and elicited robust dose-dependent and age-dependent B- and T-cell responses. Transient mild/moderate injection site pain, fever, chills, fatigue and headache were more common after the second dose. Four weeks after the second dose, in the adult cohort, MicroVNT-50 geometric mean titre against wild-type SARS-CoV-2 was 848 (95% CI, 483–1,489), 736 (459–1,183) and 1,140 (854–1,522) IU ml−1 at 10, 25 and 50 μg doses, respectively, versus 285 (196–413) IU ml−1 for human convalescent sera. All dose levels elicited 100% seroconversion, with geometric mean titre ratios 4–8-fold higher than for human convalescent sera (P < 0.01), and high IFNγ spot-forming cells per million peripheral blood mononuclear cells. The 50 μg dose induced better cross-neutralization against Alpha, Beta, Gamma and Delta variants than lower doses. ChulaCov19 at 50 μg is well tolerated and elicited higher neutralizing antibodies than human convalescent sera, with strong T-cell responses. These antibodies cross-neutralized four variants of concern. ChulaCov19 has proceeded to phase 2 clinical trials. We conclude that the mRNA vaccine expressing a prefusion non-stabilized spike protein is safe and highly immunogenic.
Similar content being viewed by others
Main
More than 10 billion doses of COVID-19 vaccines have been administered worldwide but only 10% of those living in low-income countries have received at least one dose (8 February 2022)1. Among approved vaccines, mRNA vaccines have the highest efficacy2. Building capacity to develop and manufacture mRNA vaccines in low- to middle-income countries (LMIC) is important for the current and future pandemics. The ChulaCov19 mRNA vaccine (ChulaCov19) is a lipid nanoparticle (LNP)-encapsulated nucleoside-modified mRNA encoding a non-stabilized SARS-Cov-2 spike protein. This spike protein consists of an extracellular domain with no transmembrane and cytoplasmic domains; therefore, it will be a secreted spike protein. Two major differences between ChulaCov19 and the Pfizer/BioNTech and Moderna vaccines are that ChulaCov19 is not prefusion stabilized and it is encapsulated in a different LNP formulation.
One of the major constraints for LMICs in developing and manufacturing mRNA vaccines is the patent rights protection and licensing issue. In most instances, companies that own or otherwise control COVID-19 vaccines approved for clinical use have retained licensing and control over vaccine production. To overcome this obstacle, we adopted a different payload strategy, encoding a prefusion non-stabilized form of the spike protein with an alternate lipid nanoparticle composition.
Besides this vaccine being more economically feasible for LMICs to implement, we also tested its stability at 2–8 °C. ChulaCov19 was designed in Thailand and manufactured in North America for early clinical trials, in parallel with large-scale production capacity development in Thailand. In rodent and macaques, ChulaCov19 elicited strong B- and T-cell responses3.
Results
Enrolment and follow-up
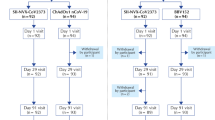
Between 28 May 2021 and 2 July 2021, 132 individuals were screened (36 were enroled in each age cohort, with 12 per dose group in each age cohort) (Extended Data Figs. 1 and 2). All but one participant completed both vaccinations. The participant declining a second vaccination was from the adult 25 μg group and experienced moderate myalgia after the first vaccination, which resolved within 3 d and was accompanied by moderate creatinine phosphokinase elevation but normal cardiac enzymes, possibly related to strenuous physical labour. This participant was excluded from the per protocol immunogenicity analysis, as were two others: one participant from the adult 10 μg group who was diagnosed with asymptomatic COVID-19 on the sixth day after the second vaccination and one participant from the adult 50 μg group who had confirmed positive baseline anti-receptor binding domain (RBD) binding antibody test but was negative for SARS-CoV-2 molecular testing, indicating asymptomatic COVID-19 before enrolment. All participants contributed to the safety analysis.
Demographic and baseline clinical characteristics
Characteristics of the study participants are listed in Table 1. Mean ages(s.d.) in the adult and elderly cohorts were 35.8 (9.1) and 65.1 (5.4) yr, respectively; body mass indices were 23 (2.8) and 23.3 (3.3), and proportions of females were 67% and 53%, respectively.
Safety and tolerability of ChulaCov19
The most common local reaction was injection site pain. Its incidence was dose-dependent, more common in adults than the elderly, and after Dose 2. All local reactions were mild in the elderly; some moderate events occurred in adults. All participants recovered after 2.79 ± 1.7 and 1.91 ± 0.9 d on average in adults and the elderly after Dose 2, respectively. A single event of severe erythema in the adult 10 μg group after Dose 2 resolved within 6 d (Fig. 1).
a,b, Severity of solicited local and systemic reactions in the adult cohort (18–55 yr old) (a) and the elderly cohort (56–75 yr old) (b). Data on local and systemic reactions were collected using paper diaries for 7 d after each vaccination. Injection-site (local) reactions were: pain at injection site (mild: does not interfere with activity; moderate: interferes with activity; severe: prevents daily activity; and grade 4: emergency room visit or hospitalization), redness and swelling (mild: 2.5–5.0 cm in diameter; moderate: 5.1–10.0 cm in diameter; severe: >10.0 cm in diameter; and grade 4: necrosis or exfoliative dermatitis). Solicited systemic events were: fever (mild: 38.0–38.4 °C; moderate: 38.5–38.9 °C; severe: 39.0–40.0 °C; and grade 4: >40.0 °C), chills, headache, fatigue, myalgia and arthralgia (all were graded as mild and did not interfere with the daily activities of the participants), moderate (interferes with daily activities), severe (prevents daily activities) or grade 4 (led to an emergency department visit or hospitalization). The numbers above the bars show the overall percentage of the participants in each group who reported the specified systemic event.
The three most common systemic reactions were fever, headache and fatigue. Systemic reactions were more common at the 25 μg and 50 μg doses, after Dose 2, and in adults. Most reactions were mild to moderate and transient, with mean duration of 1.97 ± 1.2 d in adults and 1.39 ± 0.5 d in the elderly after Dose 2 (Fig. 1).
Immunogenicity of ChulaCov19
SARS-CoV-2 RBD antibody responses
In the adult cohort 4 weeks after the second vaccination (day 50), the geometric mean titre (GMT) of anti-RBD antibody was significantly higher than for human convalescent sera 4 weeks after COVID-19 diagnosis (Fig. 2a), with geometric mean ratios (GMR) of 3.78 (95%CI 1.64–8.68), 3.23 (1.41–7.42) and 7.74 (3.37–17.77), respectively (P ≺ 0.01) (Supplementary Table 10). In the elderly cohort, anti-RBD GMTs were also dose-dependent but lower than observed in the adult cohort (Fig. 2a and Supplementary Table 6).
a, The GMTs of anti-RBD antibody measured by ELISA. b, The GMTs of IgG antibody against S trimer protein of SARS-CoV-2 measured by ELISA. c, The GMTs of neutralizing antibody against wild-type SARS-CoV-2 (Micro-VNT50). *Fold-difference as compared to the adults’ GMT, **Comparison of GMTs of 50 μg dose in adults at day 29. d, The GMTs of pseudovirus neutralizing antibody against wild-type SARS-CoV-2 (PsVNT-50). The right side of a, c and d indicate two reference values from 27 Pfizer/BioNTech mRNA Malaysian vaccinees. The serum samples were collected on day 29 after the first dose. Thirty HCS were collected at 4 weeks after confirmed diagnosis. Error bars indicate 95% confidence intervals. The numbers above the bars show the GMT of each group.
In the adult cohort at day 50, ChulaCov19 at 50 μg elicited the highest anti-spike GMT at 24,493.9 binding antibody units ml−1 (P < 0.01). In the elderly cohort, the anti-spike GMT was lower than that of the adults at all three doses, while anti-spike GMT for the 50 μg dose was significantly higher than for the 10 μg dose (P < 0.001, Fig. 2b). GM of % RBD-ACE2 binding inhibition showed a dose-dependent pattern in both age cohorts at day 29 but not at day 50. All doses at day 50 in both age cohorts elicited GM > 90% inhibition. Inhibition (%) of the human convalescent serum (HCS) and Pfizer/BNT vaccinee’s (day 29) panels were 76% and 93%, respectively (Supplementary Fig. 1).
SARS-CoV-2-specific live-virus micro-neutralization tests (MicroVNT-50)
Seroconversion rate on MicroVNT-50 in all dose groups reached 100% by day 50. GMT of the responses against wild-type (WT) virus were both dose- and age-dependent, and markedly increased from day 29 to day 50 for the 10, 25 and 50 μg doses (Fig. 2c). In the adult cohort at day 50, ChulaCov19 at 10, 25 and 50 μg doses induced significantly higher MicroVNT-50 GMT than HCS at a ratio of 2.98 (95%CI 1.63–5.44), 2.59 (1.42–4.72) and 4.01 (2.2–7.31), respectively (P < 0.01), whereas in the elderly cohort the values were 0.48 (0.25–0.92), 1.26 (0.67–2.36) and 2.09 (1.12–3.91), respectively (Supplementary Table 7). ChulaCov19 at the 50 μg dose induced significantly higher MicroVNT-50 GMT against WT and 3 variants compared with the 10 μg dose in both age cohorts, while elicited MicroVNT-50 GMTs were lower for all doses in the elderly cohort compared with the adult cohort (Fig. 3a and Supplementary Table 8).
The serum samples were collected on day 29 after the first dose from participants in each cohort (adult n = 11 per dose, elderly n = 12 per dose). The numbers above the bars show the GMT in the group. Error bars indicate 95% confidence intervals. a, The GMTs of neutralizing antibody against the SARS-CoV-2 VOCs Alpha, Beta and Delta. b, The GMTs of pseudovirus neutralizing antibody against SARS-CoV-2 VOCs Alpha, Beta, Gamma and Delta. The right side of b indicates the reference values from 27 Pfizer/BioNTech mRNA Malaysian vaccinees. The serum samples were collected on day 29 after the first dose.
Pseudovirus neutralization tests (PsVNT-50)
In the adult cohort, PsVNT-50 GMTs (95% CI) of ChulaCov19 against WT at day 50 for 10, 25 and 50 μg and for the HCS panel were 885.3 (95%CI, 438.2–1,788.7), 902.9 (445.5–1,829.7), 1,273.1 (874.2–1,854) and 471.2 (290.5–764.5), respectively (Fig. 2d). The GMT ratio of 50 μg dose/HCS was 2.7 (P = 0.01). At day 29, ChulaCov19 at 50 μg dose induced significantly higher GMT than the Pfizer/BNT vaccinated panel against Alpha, Beta and Gamma variants at a ratio of 4.47, 11.94 and 4.23 (P = 0.01), respectively (Fig. 3b and Supplementary Table 9). PsVNT-50 test results for a new variant, Omicron, showed that two doses of ChulaCov19 at 50 mg elicited a substantial decline in GMT against the Omicron variant compared with WT (Supplementary Fig. 2).
SARS-Cov-2 spike-specific T-cell responses
At day 29, all participants in the adult and elderly cohorts at 10, 25 and 50 μg showed strong spike-specific T-cell responses measured by IFNγ-ELISpot tests. The responses were lower in the elderly at 10 and 25 μg than in adults (Fig. 4a and Supplementary Table 11). In adults, both spike-specific IFNγ+CD4+ and IL2+CD4+ T-cell percentages were notably higher in the 25 and 50 μg dose recipients compared with the 10 μg dose (Supplementary Table 12). ChulaCov19 elicited spike-specific Th1-dominated responses (Fig. 4b).
PBMCs were obtained on day 29 after the first dose from participants in each cohort (adult n = 11 per dose, elderly n = 12 per dose). The numbers above the bars show the GMT in the group. Error bars indicate 95% confidence intervals. a, SARS-Cov-2 wild-type spike-specific IFN-γ ELISPOT T cells. *Comparison of the adults’ GMTs shown as fold-difference. b, SARS-CoV-2 spike-protein-specific CD4+ and CD8+ T-cell responses, and Th1/Th2 polarization responses quantified by intracellular cytokine staining.
Vaccine stability
We assessed ChulaCov19 appearance, pH, osmolality and lipid content under varying storage conditions. The key parameters related to vaccine stability are particle size, mRNA integrity and mRNA encapsulation. We detected no substantial change in these parameters at −75 °C for up to 9 months, at −20 °C for up to 6 months, or at 2–8 °C for up to 3 months. However, there was a small increase in particle size (72, 76 vs 78 nm, respectively; nevertheless, all remained within the acceptance criteria of 50–120 nm) when vaccine was stored at 2–8 °C at 6 months. Moreover, the mRNA integrity further decreased after 6 months at 2–8 °C, to 54% as compared to at −20 °C. Thus, all parameters remained in line with the vaccine specification at 6 months for all storage conditions and there were no substantial changes for any parameter tested at −75 °C and −20 °C. There were no remarkable changes in any parameter after 3 months storage at 2–8 °C and small changes after 6 months, but all the results remained within specification and where specifications are not set, the changes were minimal and would not be expected to have any impact on the functionality of the LNP (although note that this was not tested). These conclusions will be verified by future stability studies to be performed in future clinical development (Supplementary Table 15).
Discussion
To increase access to effective COVID-19 mRNA vaccines in LMICs, and to be prepared for this and any future viral pandemics, complete mRNA-LNP vaccine development and manufacturing value chains need to be established in these countries. Unlike currently approved mRNA vaccines, such as Pfizer/BNT and Moderna4, ChulaCov19 is a wild-type non-stabilized spike-protein-encoded mRNA vaccine encapsulated with a different LNP that is thermostable at 2–8 °C for at least 3 months. In a phase 1 trial, three dosages of ChulaCov19 vaccine were well tolerated, with no serious adverse effects observed in either age-group. Injection site pain was the most common adverse effect, while fever, chills, headache and myalgia were reported to be dose-dependent and more frequent after the second dose. Adverse effects were both less frequent and milder among the elderly participants. Moderate to severe events were rare overall and all adverse effects resolved on average within 2.5 d.
The results indicate that this LNP-encapsulated non-di-Proline-stabilized spike-protein mRNA vaccine is strongly immunogenic. ChulaCov19 at 50 μg dose induced high SARS-CoV-2-binding and neutralizing antibodies 1 week after dose 2 (P < 0.01), with a microVNT-50 GMT 6-fold higher than those of HCS. At 4 weeks after dose 2, there was a further rise in all tested antibodies and all 3 doses elicited microVNT-50 GMTs higher than those of HCS, with a GMT ratio that is 4–8-fold (P < 0.01). The 50 μg dose induced higher microVNT-50 and psVNT-50 GMTs against tested variants than the lower doses (P < 0.01). In the adult cohort, at 1 week after dose 2, the 50 μg dose elicited psVNT-50 GMTs against Alpha, Beta and Gamma variants at ratios that were 4.5, 11.9 and 4.2, respectively, which are higher than those of the Pfizer/BNT vaccine (P < 0.01). In terms of the Omicron variant, as reported in approved Covid-19 mRNA vaccines, two doses of the ChulaCov19 vaccine may not be effective and a third dose is required5. Developing pan-SARS-Cov-2 or pan-Coronavirus vaccine against future pandemics is warranted6.
All doses of ChulaCov19 generated strong T-cell responses and the higher doses (25 and 50 μg) elicited higher % of spike-specific IL2+CD4+ T cells and % of IFNγ+CD4+ T cells than the 10 μg dose (P < 0.01 and P < 0.05, respectively). At all doses, ChulaCov19 elicited predominantly SARS-CoV-2-specific Th1-type responses. On the basis of these results, the Data Safety Monitoring Board (DSMB) has recommended that ChulaCov19 be further advanced to a phase 2 randomized-controlled trial with the 50 μg dose.
This vaccine did not use prefusion stabilization of spike with two prolines (K986P/V987P), which many approved COVID-19 vaccine platforms use7. The phase I data presented here show that ChulaCov19 elicited robust humoral and T-cell responses, which were often greater than those of the BioNTech/Pfizer vaccine that contains the di-proline modification. There are other differences in the BioNTech/Pfizer modified mRNA vaccine that could account for the greater potency of ChulaCov19, including different untranslated regions, coding sequence optimization, poly(A) tail and the LNP formulation used. Our data support the idea that the spike does not need to be prefusion stabilized to induce a potent and protective response, unlike respiratory syncytial virus8.
Previous studies suggest that a GMT ratio of neutralizing or binding antibody in vaccinees against wild-type virus to the level in convalescent patients of >1 is associated with >70% vaccine efficacy rate2. In addition, higher binding and neutralizing antibody levels at 4 weeks after the second dose were found to correlate with symptomatic infection risk reduction in the AZD1222 trial9 and COVE study10. ChulaCov19 induced strong binding and neutralizing antibody responses 4 weeks after the second dose, and the ratio of neutralizing antibody GMT in ChulaCov19 vaccinees vs convalescent sera of 4 weeks after diagnosis of approximately 4–8-fold suggests that this vaccine candidate has a potentially significant vaccine efficacy. A consensus on correlates of protection may be a challenge11 and recently the International Coalition of Medicines Regulatory Authorities has accepted well-designed immunobridging studies for authorizing COVID-19 vaccines12. However, due to escape of the Omicron variant from current approved vaccines, the efficacy of all current vaccines is reduced and a new variant-specific analysis is therefore required13,14,15. One of the limitations of currently approved mRNA COVID-19 vaccines is that recent data have shown that the neutralizing antibody (NAb) against WT and Omicron induced by 2 doses of approved COVID-19 vaccines wane over time. In addition, due to a greater vaccine escape of Omicron variants, the cross-neutralizing titre against the Omicron variant is several folds lower than against WT, therefore the decline rate is much faster15. Administering a third dose of COVID-19 mRNA vaccine as a booster has enabled a substantial increase in GMT against WT, Delta and Omicron variants16. In our study, administration of 2 doses of ChulaCov19 vaccine induced neutralizing antibodies with less potency for the Omicron variant, although only mild-to-moderate reductions in efficacy against other variants of concern (VOCs) were observed (Fig. 3). It is therefore likely that dual vaccinations with ChulaCov19 vaccine may need a third booster dose, ideally with a second-generation vaccine.
Accessibility to effective COVID-19 vaccines, particularly mRNA vaccines, remains very limited in many LMICs17, and the main goal of ChulaCov19 development is to be part of the solution to this problem. There are several challenges to overcome: clinical development to reach emergency use authorization (EUA), establishing large-scale manufacturing capacity, negotiation of expanding LNP-licensing territory, and the cost and long lead-time of raw materials. BioNet Asia, Thailand has already established manufacturing capacity for both mRNA production and encapsulation, and a first clinical lot has been released for further clinical development and EUA approval.
The limitations of this study include the small sample size and dose-finding design. The exploratory comparative immunogenicity analyses with convalescent sera or Pfizer/BNT vaccinees’ sera are not head-to-head comparisons and are not free from bias. Convalescent sera were collected during the Delta wave in Thailand, and it is possible that human convalescent sera antibody responses are stronger against Delta than against WT. To minimize bias, the convalescent and Pfizer/BNT vaccinees’ serum samples were tested at the same laboratories together with the ChulaCov19 vaccinated samples. In addition, an randomized controlled trial phase 2 study has begun and a larger-scale immune-bridging, non-inferiority phase 3 study is planned.
In summary, we provide presumably the first evidence in humans that an mRNA vaccine expressing a prefusion non-stabilized spike protein is safe and highly immunogenic, similar to an approved mRNA vaccine, Pfizer/BNT, expressing a prefusion spike protein stabilized by the addition of a di-proline mutation. ChulaCov19 mRNA vaccine is well tolerated, elicited strong SARS-CoV-2-specific B- and T-cell immunogenicity, and is currently under phase 2 and later clinical development.
Methods
Trial design
This phase 1, open-label, dose-escalation study to evaluate safety and immunogenicity of ChulaCov19 vaccine enroled healthy participants aged 18–55 (adults, n = 36), followed by a cohort aged 56–75 (elderly, n = 36). The inclusion and exclusion criteria are shown in the Supplementary Table 1. All participants meeting eligibility criteria were randomized sequentially in a 1:1:1 allocation ratio into each dose schedule. Both age cohorts received ChulaCov19 at 10, 25 or 50 µg per dose, with 12 participants per dose group for each age cohort, in a sentinel dose-escalating manner.
The trial and the Investigational New Drug application were approved by the ethics committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, and Thailand’s Food and Drug Administration, respectively. All participants provided written informed consent. The trial was conducted at the Chula Clinical Research Center and King Chulalongkorn Memorial Hospital, Bangkok.
Trial vaccine
ChulaCov19 mRNA was manufactured at Trilink Biotechnologies (San Diego, California), and the mRNA-LNP vaccine was manufactured at Integrity Bio Inc. (Camarillo, California). The vaccine consists of ChulaCov19 mRNA encapsulated in a proprietary LNP delivery system developed by Genevant Sciences Corporation (Vancouver, British Columbia). ChulaCov19 was stored as a sterile suspension of 0.2 mg ml−1 at −75 ± 10 °C and diluted with normal saline according to the assigned dose, to be given at 0.5 ml intramuscularly (IM).
Trial procedures
Four adult sentinel participants were enroled to receive ChulaCov19 at 10 µg IM. Once no halting criteria (Supplementary Table 2) were reported by day 3, the remaining eight 10 µg participants were enroled. The same approach was followed for the 25- and 50 μg doses. The vaccine was administered in the deltoid muscle on day 1 and day 22 ± 3, followed by a 2 h safety monitoring on-site. Enrolment of the elderly participants was commenced after DSMB review of the data when the last adult participant of the 10 µg group had reached day 29 (1 week after the second vaccination) on study. A diary was provided to participants to record solicited and unsolicited adverse reactions, and concomitant medications for 7 d. Safety laboratory tests were performed at baseline and days 8, 22, 29 and 50.
Assessment of safety and tolerability
Safety endpoints included solicited and unsolicited local adverse events, systemic adverse events, use of antipyretics/analgesics in the 7 d after vaccination, and serious adverse events up to day 50 (4 weeks after the second vaccination). Castor EDC version 2021.2 was used for data collection.
Assessment of immunogenicity
Binding to the SARS-CoV-2 S1 RBD was measured by enzyme-linked immunosorbent assay (ELISA). Neutralizing antibody titres against WT and VOCs were assessed by MicroVNT-50 and PsVNT-50. SARS-Cov-2 RBD-ACE2 blocking antibody was measured by surrogate viral neutralization test. Cellular immunity was measured by IFNγ-ELISpot assay (ELISpot) and by intracellular cytokine staining (ICS) assay (Supplementary Table 3). Tests were performed on specimens collected at days 1, 8, 22, 29 and 50; however, PsVNT-50 was performed only at days 29 and 50. Exploratory comparator serum panels included (1) 30 HCS from adults with median age (s.d.) of 39.9 (16.6) years, 63.3% of whom were female and (2) 27 Pfizer/BioNTech mRNA vaccinees. Genotypic testing for the virus variant among human convalescents’ sera was not available. However, based on the epidemiology data, it is likely to be wild-type SARS-Cov-2. The serum samples were collected at 4 weeks after confirmed diagnosis. All Pfizer/BioNTech mRNA vaccinees were Malaysian with median age (s.d.) of 34.5 (9.4) years, 77.8% of whom were female. Serum samples were collected at day 29 after the first dose (Supplementary Tables 4 and 5).
Assessment of vaccine stability
Samples of the ChulaCov19 vaccine cinical lots were stored at 3 different temperatures: −75 °C ± 10 °C, −20 °C ± 5 °C, 5 °C ± 3 °C. Samples were analysed at planned timepoints 1, 3, 6, 9, 12, 18 and 24 months; 3, 6 and 12 months; and 3 and 6 months, respectively, for each temperature. The ongoing study is conducted at Intertek Pharmaceutical Services Laboratory in Manchester, UK (Supplementary Tables 13 and 14)
Statistical analysis
Sample size for phase I was based on practical and medical considerations rather than power for statistical hypothesis testing or precision of parameter estimation. Results of safety analyses are presented as counts and percentages, and group ages as mean (s.d.). Summary descriptive statistics relevant for study endpoints were provided for each cohort and vaccine dose group at the study timepoints indicated in the protocol.
Immunogenicity was analysed per protocol. GMTs and their 95% CIs were calculated for MicroVNT-50, PsVNT-50, anti-RBD-IgG, anti-S Trimer, sVNT, IFNγ-ELISpot and Th1/Th2 spike-specific CD4+ and CD8+ T cells. GMTs were calculated as exponentiated means of logarithmic-transformed assay results. Formal comparisons of MicroVNT-50, PsVNT-50, anti-RBD-IgG and sVNT against samples of 30 HCS from adults with COVID-19 and 27 Pfizer/BioNTech mRNA vaccinees were made using GMR (95% CI). The unadjusted and age-adjusted GMRs and 95% CIs were exponentiated with coefficients from the linear regression models, with natural log-transformed titres as outcome variables. Statistical analysis was conducted with Stata 17, and all statistical tests were two-sided.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Coronavirus Pandemic (COVID-19) Vaccinations (Our World in Data, 2020).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Prompetchara, E. et al. Immunogenicity and protective efficacy of a sars-cov-2 mrna vaccine encoding secreted non-stabilized spike protein in mice. Preprint at bioRxiv https://doi.org/10.1101/2022.09.07.506878 (2022).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Thompson, M. G. et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb. Mortal. Wkly Rep. 71, 139–145 (2022).
Saunders, K. O. et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature 594, 553–559 (2021).
Martínez-Flores, D. et al. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front. Immunol. 12, 701501 (2021).
McLellan, J. S. et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598 (2013).
Feng, S. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 27, 2032–2040. (2021).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. Science 375, 43–50 (2022).
Koup, R. A. et al. A government-led effort to identify correlates of protection for COVID-19 vaccines. Nat. Med. 27, 1493–1494 (2021).
Access Consortium: Alignment with ICMRA Consensus on Immunobridging for Authorising New Covid-19 Vaccines. (Access Consortium, 2021).
Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656 (2022).
Schmidt, F. et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N. Engl. J. Med. 386, 599–601 (2021).
Sievers, B. L. et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses. Sci. Transl. Med. 14, eabn7842 (2022).
Barda, N. et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 398, 2093–2100 (2021).
Mathieu, E. et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 5, 947–953 (2021).
Acknowledgements
This study was funded by National Vaccine Institute (NVI) grant no. 2563.1/11 and 2564.1/4; C2F Fund-Chulalongkorn Academic Advancement into Its 2nd Century Project (CUAASC); the Ratchadapisek Sompoch Endowment Fund (2021), Chulalongkorn University (764002-HE04); and Public Donation through Covid-19 vaccine development fund of the Faculty of Medicine, Chulalongkorn University and the Thai Red Cross Society, Thailand. We thank all the study participants without whose support the study would not have been possible.
Author information
Authors and Affiliations
Consortia
Contributions
S.G., W.K. and K.R. managed the participants, developed the strategy plan, interpreted data, drafted and revised the manuscript. M.-G.A., W.W., A.C. and L.H. provided consultation for the investigational product and revised the manuscript. C.K., E.P., A.T., A.J., S.B., S.U. and T. Palaga performed the experiments and revised the manuscript. J.S., T.A. and S.K. performed the statistical analysis and revised the manuscript. A.K. assisted with the study design, provided reference samples and revised the manuscript. S.S., T. Puthanakit and K.P. managed the participants, developed the strategy plan and revised the manuscript. E.K. drafted and revised the manuscript. D.W. and K.R. conceptualized the work and strategy, provided consultation for the investigational product and revised the manuscript. K.R. conceptualized the work and strategy, supervised the study trial and revised the manuscript. All authors read and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that Drew Weissman is named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins and vaccines. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from licensing of our patents. The other authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Thiago Cerqueira-Silva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Participants flow diagram of the adult cohort.
All the participants received two doses of the ChulaCov19 vaccine, except for one participant who was assigned to receive 25 μg of ChulaCov19, who received one dose.
Extended Data Fig. 2 Participants flow diagram of the elderly cohort.
All the participants received two doses of the ChulaCov19 vaccine.
Supplementary information
Supplementary Information
ChulaVAC-001 Study Team and affiliations, Study inclusion and exclusion criteria, Study halting criteria, Immunogenicity laboratory methods, Demographic characteristics of human convalescent serum samples, Demographic characteristics of Pfizer/BioNTech mRNA vaccinees, Supplementary Figs. 1 and 2, and Tables 1–13.
Supplementary Data
ChulaCov001 protocol and amendments.
Source data
Source Data Fig. 1
Local and systemic adverse events, by dose group and age cohorts.
Source Data Fig. 2
Immunogenicity assay responses to ChulaCov.
Source Data Fig. 3
SARS-CoV-2 neutralization responses against variants of concern.
Source Data Fig. 4
Magnitude of ChulaCov-induced CD4+ and CD8+ T-cell responses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gatechompol, S., Kittanamongkolchai, W., Ketloy, C. et al. Safety and immunogenicity of a prefusion non-stabilized spike protein mRNA COVID-19 vaccine: a phase I trial. Nat Microbiol 7, 1987–1995 (2022). https://doi.org/10.1038/s41564-022-01271-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-022-01271-0
This article is cited by
-
Phase II prefusion non-stabilised Covid-19 mRNA vaccine randomised study
Scientific Reports (2024)