Abstract
The innate immune system fights infection with inflammasomes and interferons. Facultative bacterial pathogens that inhabit the host cytosol avoid inflammasomes1,2,3,4,5,6 and are often insensitive to type I interferons (IFN-I), but are restricted by IFN-γ7,8,9,10,11. However, it remains unclear how obligate cytosolic bacterial pathogens, including Rickettsia species, interact with innate immunity. Here, we report that the human pathogen Rickettsia parkeri is sensitive to IFN-I and benefits from inflammasome-mediated host cell death that antagonizes IFN-I. R. parkeri-induced cell death requires the cytosolic lipopolysaccharide (LPS) receptor caspase-11 and antagonizes IFN-I production mediated by the DNA sensor cGAS. The restrictive effects of IFN-I require the interferon regulatory factor IRF5, which upregulates genes encoding guanylate-binding proteins (GBPs) and inducible nitric oxide synthase (iNOS), which we found to inhibit R. parkeri. Mice lacking both IFN-I and IFN-γ receptors succumb to R. parkeri, revealing critical and overlapping roles for these cytokines in vivo. The interactions of R. parkeri with inflammasomes and interferons are similar to those of viruses, which can exploit the inflammasome to avoid IFN-I12, are restricted by IFN-I via IRF513,14, and are controlled by IFN-I and IFN-γ in vivo15,16,17. Our results suggest that the innate immune response to an obligate cytosolic bacterial pathogen lies at the intersection of antibacterial and antiviral responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The RNA-seq datasets generated and analysed during this study are available in the Gene Expression Ombibus (GEO) repository, accession no. GSE128211. R. parkeri strains were authenticated by whole-genome sequencing and are available in the National Center for Biotechnology Information (NCBI) Trace and Short-Read Archive; Sequence Read Archive (SRA), accession no. SRX4401164. Source Data for Figs. 1–4 and Extended Data Figs. 3 and 5 are provided with the paper.
References
Shen, A. & Higgins, D. E. The MogR transcriptional repressor regulates nonhierarchal expression of flagellar motility genes and virulence in Listeria monocytogenes. PLoS Pathog. 2, e30 (2006).
Sauer, J.-D. et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7, 412–419 (2010).
Sauer, J.-D. et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc. Natl Acad. Sci. USA 108, 12419–12424 (2011).
Wallet, P., Lagrange, B. & Henry, T. in Inflammasome Signaling and Bacterial Infections. Current Topics in Microbiology and Immunology Vol. 397 (ed. Backert, S.) 229–256 (Springer, 2016).
Theisen, E. & Sauer, J.-D. Listeria monocytogenes and the inflammasome: from cytosolic bacteriolysis to tumor immunotherapy. Curr. Top. Microbiol. Immunol. 397, 133–160 (2016).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Henry, T. et al. Type I IFN signaling constrains IL-17A/F secretion by γδ T cells during bacterial infections. J. Immunol. 184, 3755–3767 (2010).
Auerbuch, V., Brockstedt, D. G., Meyer-Morse, N., O’Riordan, M. & Portnoy, D. A. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200, 527–533 (2004).
Woodward, J. J., Iavarone, A. T. & Portnoy, D. A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328, 1703–1705 (2010).
Billiau, A. & Matthys, P. Interferon-γ: a historical perspective. Cytokine Growth Factor Rev. 20, 97–113 (2009).
Rayamajhi, M., Humann, J., Penheiter, K., Andreasen, K. & Lenz, L. L. Induction of IFN-αβ enables Listeria monocytogenes to suppress macrophage activation by IFN-γ. J. Exp. Med. 207, 327–337 (2010).
Wang, Y. et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity 46, 393–404 (2017).
Thackray, L. B. et al. Interferon regulatory factor 5-dependent immune responses in the draining lymph node protect against West Nile virus infection. J. Virol. 88, 11007–11021 (2014).
Proenca-Modena, J. L. et al. Interferon-regulatory factor 5-dependent signaling restricts orthobunyavirus dissemination to the central nervous system. J. Virol. 90, 189–205 (2016).
Milligan, G. N. et al. A lethal model of disseminated dengue virus type 1 infection in AG129 mice. J. Gen. Virol. 98, 2507–2519 (2017).
Rossi, S. L. et al. Characterization of a novel murine model to study zika virus. Am. J. Trop. Med. Hyg. 94, 1362–1369 (2016).
Müller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Stetson, D. B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373–381 (2006).
Schoenborn, J. R. & Wilson, C. B. Regulation of interferon‐γ during innate and adaptive immune responses. Adv. Immunol. 96, 41–101 (2007).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Banerjee, I. et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49, 413–426 (2018).
Liu, B. C. et al. Constitutive interferon maintains GBP expression required for release of bacterial components upstream of pyroptosis and anti-DNA responses. Cell Rep. 24, 155–168 (2018).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Storek, K. M., Gertsvolf, N. A., Ohlson, M. B. & Monack, D. M. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J. Immunol. 194, 3236–3245 (2015).
Paddock, C. D. et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 47, 1188–1196 (2008).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Turco, J., Liu, H., Gottlieb, S. F. & Winkler, H. H. Nitric oxide-mediated inhibition of the ability of Rickettsia prowazekii to infect mouse fibroblasts and mouse macrophagelike cells. Infect. Immun. 66, 558–566 (1998).
Feng, H. M. & Walker, D. H. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect. Immun. 68, 6729–6736 (2000).
Turco, J. & Winkler, H. H. Role of the nitric oxide synthase pathway in inhibition of growth of interferon-sensitive and interferon-resistant Rickettsia prowazekii strains in L929 cells treated with tumor necrosis factor alpha and gamma interferon. Infect. Immun. 61, 4317–4325 (1993).
Walker, D. H., Popov, V. L., Crocquet-Valdes, P. A., Welsh, C. J. & Feng, H. M. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab. Invest. 76, 129–138 (1997).
Yamamoto, M. et al. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 37, 302–313 (2012).
Maltez, V. I. et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 43, 987–997 (2015).
Robinson, N. et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat. Immunol. 13, 954–962 (2012).
Stanley, S. A., Johndrow, J. E., Manzanillo, P. & Cox, J. S. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178, 3143–3152 (2007).
Hedges, J. F. et al. Type I interferon counters or promotes Coxiella burnetii replication dependent on tissue. Infect. Immun. 84, 1815–1825 (2016).
Qiu, H. et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 181, 2092–2102 (2008).
de Almeida, L. A. et al. MyD88 and STING signaling pathways are required for IRF3-mediated IFN-β induction in response to Brucella abortus infection. PLoS ONE 6, e23135 (2011).
Engström, P. et al. Evasion of autophagy mediated by Rickettsia surface protein OmpB is critical for virulence. Nat. Microbiol. 4, 2538–2551 (2019).
Man, S. M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015).
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Mariathasan, S., Weiss, D. S., Dixit, V. M. & Monack, D. M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049 (2005).
Boxx, G. M. & Cheng, G. The roles of type I interferon in bacterial infection. Cell Host Microbe 19, 760–769 (2016).
Darby, A. C., Cho, N.-H., Fuxelius, H.-H., Westberg, J. & Andersson, S. G. E. Intracellular pathogens go extreme: genome evolution in the Rickettsiales. Trends Genet. 23, 511–520 (2007).
MacMicking, J. D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12, 367–382 (2012).
Fritsch, S. D. & Weichhart, T. Effects of interferons and viruses on metabolism. Front. Immunol. 7, 630 (2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Edelstein, A. D. et al. Advanced methods of microscope control using μManager software. J. Biol. Methods 1, e10 (2014).
Jiang, Z. et al. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6, 565–570 (2005).
National Research Council. Guide for the Care and Use of Laboratory Animals 8th edn (National Academies Press, 2011).
Rauch, I. et al. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of Caspase-1 and -8. Immunity 46, 649–659 (2017).
Sauer, J.-D. et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79, 688–694 (2011).
Marcus, A. et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity 49, 754–763 (2018).
Purtha, W. E., Swiecki, M., Colonna, M., Diamond, M. S. & Bhattacharya, D. Spontaneous mutation of the Dock2 gene in Irf5 −/− mice complicates interpretation of type I interferon production and antibody responses. Proc. Natl Acad. Sci. USA 109, E898–E904 (2012).
Szretter, K. J. et al. 2′-O methylation of the viral mRNA cap by West Nile virus evades Ifit1-dependent and -independent mechanisms of host restriction in vivo. PLoS Pathog. 8, e1002698 (2012).
Fensterl, V. et al. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 8, e1002712 (2012).
Ishida, T. et al. Endothelial lipase is a major determinant of HDL level. J. Clin. Invest. 111, 347–355 (2003).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Matsuyama, T. et al. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 75, 83–97 (1993).
Huang, S. et al. Immune response in mice that lack the interferon-gamma receptor. Science 259, 1742–1745 (1993).
Acknowledgements
We thank J. Coers (Duke University) for femurs from Gbpchr3−/− mice. We thank M. Diamond (Washington University, St. Louis) for femurs from Irf5−/−, Ifit1−/− and Ifit2−/− mice. We thank D. Rader (the University of Pennsylvania) for femurs from LipG−/− mice. We thank E. Harris (University of California, Berkeley) for AG129 mice (originally obtained from M. Aguet, Swiss Institute for Experimental Research). We thank G. Barton (University of California, Berkeley) for advice and for Irf3−/−Irf7−/− mice and Tnfrsf1a−/−Tnfrsf1b−/− mice. We thank D. Raulet (University of California, Berkeley) and C. Nicolai for Rag2−/− mice. We thank N. Fischer for the critical reading of this manuscript. P.E. was supported by postdoctoral fellowships from the Foundation Olle Engkvist Byggmästare, the Swedish Society of Medical Research (SSMF) and the Sweden–America Foundation. M.D.W. was supported by NIH/NIAID grant nos. R01 AI109044, R21 AI109270 and R21 AI138550. J.A.F. was supported by NIH/National Institute of General Medical Sciences (NIGMS) grant no. 2T34GM008612-24. R.E.V. is a Howard Hughes Medical Institute (HHMI) investigator and is supported by NIH/NIAID grant nos. AI075039 and AI063302.
Author information
Authors and Affiliations
Contributions
T.P.B. performed and analysed in vitro and in vivo experiments. P.E. provided reagents and contributed to in vivo experiments. R.A.C. contributed to the breeding of the mice. J.A.F. collected microscopy images and contributed to image analysis. T.P.B. wrote the original draft of this manuscript with guidance and edits from M.D.W. Critical reading and further edits were also provided by P.E. and R.E.V. Supervision was provided by T.P.B., R.E.V. and M.D.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
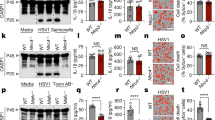
Extended Data Fig. 1 Inflammasome activation benefits R. parkeri by antagonizing the IFN-I response in mouse macrophages.
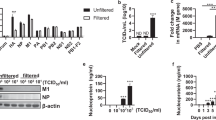
a, Measurement of Listeria monocytogenes (Lm) CFU in BMDMs, MOI of 1. 3,000 U of IFN-β added at t = 0. n = 3 independent experiments. b, Measurement of IFN-I in supernatants of WT BMDMs infected with R. parkeri (24 hpi) or L. monocytogenes (8 hpi), MOI of 1. Supernatants were used to stimulate a luciferase-expressing cell line and relative light units (RLU) were measured and compared between each sample and uninfected cells. n = 7 and 7 biological replicates. c, Time course of LDH release (blue) and IFN-I abundance as measured by RLU production (pink), in WT BMDMs infected with R. parkeri, MOI of 1. n = 3 independent experiments. d, Time course of LDH release (blue) and IFN-I abundance (pink), in Casp1/11-/- BMDMs infected with R. parkeri, MOI of 1. n = 3 independent experiments. e, Images of BMDMs infected with R. parkeri, MOI of 1, at 72 hpi. Scale bar = 100 μm. Experiments were repeated 3 times with similar results. f, Measurement of R. parkeri abundance in BMDMs, MOI of 0.2. ‘Supe’ indicates 200 μl of conditioned supernatant collected at 24 hpi from Casp1/11-/- BMDMs infected at an MOI of 1. Antibody was added at t = 0. The indicated statistical differences (*) are between WT and WT+supernatant. No statistical differences were observed between the samples treated with supernatant. g, Measurement of R. parkeri abundance in BMDMs, MOI of 1. Antibodies were added at t = 0. n = 3 independent experiments. h, Host cell death during R. parkeri infection of BMDMs. LDH release was measured at 24 hpi upon R. parkeri infection of the indicated BMDMs, MOI of 1. n = 6, 4, 4, and 4 biological replicates. Statistical comparisons in panel h were made between each sample and WT. Statistical analyses in panels a, b, f, and g used a two-tailed Student’s T-test. Statistical analyses in panel h used a one-way ANOVA with multiple comparisons and Tukey post-hoc test. For all panels: data are expressed as means and error bars represent the SD; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant.
Extended Data Fig. 2 IRF5-regulated genes, including Gbp2 and Nos2, are antirickettsial ISGs.
a, R. parkeri abundance in BMDMs. “supe” indicates 200 μl conditioned supernatant from infected Casp1/11-/- BMDMs. n = 3 independent experiments. b, qPCR of ISGs, normalized to actin. WT and mutant BMDMs were infected with R. parkeri and treated with IFN-β and RNA was analyzed at 12 hpi. Data are fold upregulation as compared to infected cells not treated with IFN-β. n = 3 separate experiments. For statistics, values were compared to the WT value for each primer set. c, R. parkeri abundance in BMDMs. “supe” indicates 200 ul conditioned supernatant from infected Casp1/11-/- BMDMs. n = 3 independent experiments. Statistical differences (*) are shown between WT and WT + supernatant. No statistical differences (ns) were observed between WT+ supernatant and mutant cells + supernatant. d, R. parkeri abundance in BMDMs. “supe” indicates 200 μl conditioned supernatant from infected Casp1/11-/- BMDMs. The L-NIL final concentration was 1 mM, added at t = 0. n = 3 independent experiments. e, Quantification of GBP2 colocalization with R. parkeri using immunofluorescence microscopy, in BMDMs, MOI of 1. Each data point is an independent experiment and includes quantification from more than 5 images totaling at least 150 bacteria. n = 3 independent experiments. Lines connect means for each time point. f, Quantification of GBP2 colocalization with R. parkeri using immunofluorescence microscopy, in BMDMs, MOI of 1 at 3 hpi. Each data point is an independent experiment and includes quantification from more than 5 images totaling at least 150 bacteria. n = 7, 7, 3, and 3 independent experiments. For experiments with exogenous IFN-I, 100 U of rIFN-β was added overnight prior to infection. Statistical analyses in panels a, b, c, d, and e used a two-tailed Student’s T-test; statistical analyses in panel f used a one-way ANOVA with multiple comparisons and Tukey post-hoc test; For all panels, data are expressed as means and error bars represent the SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant.
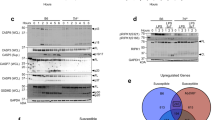
Extended Data Fig. 3 IFN-I and IFN-γ play overlapping roles in protecting against R. parkeri in vivo.
a, R. parkeri abundance in mouse organs, infected i.v. with 107 bacteria, at 72 hpi. Bars denote medians. n = 4 (control) and 5 (α-IFN-γ) individual mice, for each organ. Data are the combination of two independent experiments. Each individual data point represents an individual mouse. Statistics used a two-tailed Mann Whitney U test. *p < 0.05. b, Mouse weight after i.v. infection with 107R. parkeri. Data are normalized to the weight at t = 0. Each line represents an individual mouse. n = 5 (Ifnar-/-), 7 (Ifngr-/-), and 7 (Ifnar-/-Ifngr-/-). c, Mouse body temperature after i.v. infection with 107R. parkeri. Each line represents an individual mouse. n = 5 (Ifnar-/-), 7 (Ifngr-/-), and 7 (Ifnar-/-Ifngr-/-). d, Survival of AG129 genotype mice (lacking IFN-I and IFN-γ receptors) after i.v. infection. n = 5 (107), 7 (106), and 5 (105). Data for each group are the combination of 2 independent experiments.
Extended Data Fig. 4 Tissue necrosis, leukocyte infiltration, and vascular damage is increased in spleens and livers of infected Ifnar-/-Ifngr-/- mice.
Organs were harvested from mice intravenously infected with 107R. parkeri at 72 hpi. Samples were fixed, sliced, and stained with hematoxylin and eosin (H&E) and commercially analyzed by a pathologist for inflammation and vascular damage. Inflammation observed was infiltration of mononuclear cells including macrophages, plasma cells, and lymphocytes in both organs, and also granulocytes in the liver. Vascular changes include fibrinoid vascular wall degeneration, hypertrophy of the endothelium, perivascular fibrinous material, and fibrin thrombi in medium caliber vessels. Double-headed arrows indicate aberrations at the vasculature and single-headed arrows indicate regions of necrosis and/or regions of mononuclear infiltrates. Scale bars in the liver are 100 μm (20×), 500 μm (4×) and 1 mm (2×); scale bars in the spleen are 100 μm (20×), 200 μm (10×), and 500 μm (4×); asterisks indicate defined splenic follicles in uninfected mice, which are lost in infected Ifnar-/-Ifngr-/- mice; results were similar in 3 independent experiments.
Extended Data Fig. 5 NK and CD8+ T cells do not play a critical role in protecting against intravenous R. parkeri infection in mice.
a, R. parkeri abundance in mouse organs, infected i.v. with the indicated amounts of bacteria, at 72 hpi. Bars denote medians. From left to right, n = 4, 5, 5, and 5 individual mice, for each organ. Statistics used a two-tailed Mann Whitney U test, where each condition was compared to the WT+IgG control for each organ. *p < 0.05, **p < 0.01, and ns=not significant. b, Survival of mice after i.v. infection. n = 4 (Rag2-/-) and 5 (Ifnar-/-Ifngr-/-) individual mice. Data for each group are combined from 2 independent experiments.
Supplementary Information
Supplementary Information
Supplementary Fig. 1.
Supplementary Dataset 1
RNA-seq analysis of infected WT, Irf1-/-, Irf5-/-, and Irf3/7-/- BMDMs infected with R. parkeri.
Supplementary Table 1
Statistical source data.
Source Data Fig. 1
Statistical Source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Burke, T.P., Engström, P., Chavez, R.A. et al. Inflammasome-mediated antagonism of type I interferon enhances Rickettsia pathogenesis. Nat Microbiol 5, 688–696 (2020). https://doi.org/10.1038/s41564-020-0673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-0673-5
This article is cited by
-
A comprehensive analysis of type 1 interferon gene signatures in systematic lupus erythematosus and prediction of the crucial susceptible factor for Sjögren syndrome
Clinical and Experimental Medicine (2023)
-
A patatin-like phospholipase mediates Rickettsia parkeri escape from host membranes
Nature Communications (2022)
-
A glycine-rich PE_PGRS protein governs mycobacterial actin-based motility
Nature Communications (2022)
-
Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle
Nature Reviews Microbiology (2021)
-
Emerging technologies and infection models in cellular microbiology
Nature Communications (2021)