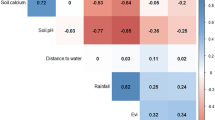
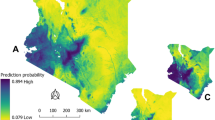
Abstract
Bacillus anthracis is a spore-forming, Gram-positive bacterium responsible for anthrax, an acute infection that most significantly affects grazing livestock and wild ungulates, but also poses a threat to human health. The geographic extent of B. anthracis is poorly understood, despite multi-decade research on anthrax epizootic and epidemic dynamics; many countries have limited or inadequate surveillance systems, even within known endemic regions. Here, we compile a global occurrence dataset of human, livestock and wildlife anthrax outbreaks. With these records, we use boosted regression trees to produce a map of the global distribution of B. anthracis as a proxy for anthrax risk. We estimate that 1.83 billion people (95% credible interval (CI): 0.59–4.16 billion) live within regions of anthrax risk, but most of that population faces little occupational exposure. More informatively, a global total of 63.8 million poor livestock keepers (95% CI: 17.5–168.6 million) and 1.1 billion livestock (95% CI: 0.4–2.3 billion) live within vulnerable regions. Human and livestock vulnerability are both concentrated in rural rainfed systems throughout arid and temperate land across Eurasia, Africa and North America. We conclude by mapping where anthrax risk could disrupt sensitive conservation efforts for wild ungulates that coincide with anthrax-prone landscapes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and approval from appropriate partner country ministries of health or agriculture.
References
Carlson, C. J. et al. Spores and soil from six sides: interdisciplinarity and the environmental biology of anthrax (Bacillus anthracis). Biol. Rev. 93, 1813–1831 (2018).
Swartz, M. N. Recognition and management of anthrax—an update. N. Engl. J. Med. 345, 1621–1626 (2001).
Anthrax in Humans and Animals (World Health Organization and International Office of Epizootics, 2008).
Alexander, K. A., Lewis, B. L., Marathe, M., Eubank, S. & Blackburn, J. K. Modeling of wildlife-associated zoonoses: applications and caveats. Vector-Borne Zoonotic Dis. 12, 1005–1018 (2012).
Hugh-Jones, M. & Blackburn, J. The ecology of Bacillus anthracis. Mol. Aspects Med. 30, 356–367 (2009).
Hugh-Jones, M. & De Vos, V. Anthrax and wildlife. Sci. Tech. Rev. Off. Int. Epizoot. 21, 359–384 (2002).
Turner, W. C. et al. Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proc. R. Soc. Lond. B 281, 20141785 (2014).
Coleman, M. E., Thran, B., Morse, S. S., Hugh-Jones, M. & Massulik, S. Inhalation anthrax: dose response and risk analysis. Biosecurity Bioterrorism 6, 147–160 (2008).
Shadomy, S. et al. Anthrax Outbreaks: A Warning for Improved Prevention, Control and Heightened Awareness (EMPRES Watch Vol. 37, FAO, 2016).
Blackburn, J. K., Kracalik, I. T. & Fair, J. M. Applying science: opportunities to inform disease management policy with cooperative research within a one health framework. Front. Public Health 3, 276 (2016).
Mullins, J. C. et al. Ecological niche modeling of Bacillus anthracis on three continents: evidence for genetic–ecological divergence? PloS ONE 8, e72451 (2013).
Chen, W.-J. et al. Mapping the distribution of anthrax in mainland China, 2005–2013. PLoS Negl. Trop. Dis. 10, e0004637 (2016).
Mullins, J. et al. Ecological niche modelling of the Bacillus anthracis A1. A sub-lineage in Kazakhstan. BMC Ecol. 11, 32 (2011).
Blackburn, J. K., McNyset, K. M., Curtis, A. & Hugh-Jones, M. E. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am. J. Trop. Med. Hyg. 77, 1103–1110 (2007).
Barro, A. S. et al. Redefining the Australian anthrax belt: modeling the ecological niche and predicting the geographic distribution of Bacillus anthracis. PLoS Negl. Trop. Dis. 10, e0004689 (2016).
Seboxa, T. & Goldhagen, J. Anthrax in Ethiopia. Trop. Geogr. Med. 41, 108–112 (1989).
Deribe, K. et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasites Vectors 5, 240 (2012).
Pieracci, E. G. et al. Prioritizing zoonotic diseases in Ethiopia using a one health approach. One Health 2, 131–135 (2016).
Griffith, J. et al. Investigation of inhalation anthrax case, United States. Emerg. Infect. Dis. 20, 280 (2014).
Kracalik, I. et al. Changing patterns of human anthrax in Azerbaijan during the post-Soviet and preemptive livestock vaccination eras. PLoS Negl. Trop. Dis. 8, e2985 (2014).
Blackburn, J. K. et al. Bacillus anthracis diversity and geographic potential across Nigeria, Cameroon and Chad: further support of a novel West African lineage. PLoS Negl. Trop. Dis. 9, e0003931 (2015).
Kracalik, I., Malania, L., Imnadze, P. & Blackburn, J. K. Human anthrax transmission at the urban–rural interface, Georgia. Am. J. Trop. Med. Hyg. 93, 1156–1159 (2015).
Hampson, K. et al. Predictability of anthrax infection in the Serengeti, Tanzania. J. Appl. Ecol. 48, 1333–1344 (2011).
Clegg, S. Preparedness for anthrax epizootics in wildlife areas. Emerg. Infect. Dis. https://doi.org/10.3201/eid1207.060458 (2006).
Turnbull, P. et al. Vaccine-induced protection against anthrax in cheetah (Acinonyx jubatus) and black rhinoceros (Diceros bicornis). Vaccine 22, 3340–3347 (2004).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
Limmathurotsakul, D. et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 1, 15008 (2016).
Kracalik, I. T. et al. Modeling the environmental suitability of anthrax in Ghana and estimating populations at risk: implications for vaccination and control. PLoS Negl. Trop. Dis. 11, e0005885 (2017).
Munang’andu, H. M. et al. The effect of seasonal variation on anthrax epidemiology in the upper Zambezi floodplain of western Zambia. J. Vet. Sci. 13, 293–298 (2012).
Lepheana, R. J., Oguttu, J. W. & Qekwana, D. N. Temporal patterns of anthrax outbreaks among livestock in Lesotho, 2005–2016. PloS One 13, e0204758 (2018).
Blackburn, J. K. et al. Modeling the ecological niche of Bacillus anthracis to map anthrax risk in Kyrgyzstan. Am. J. Trop. Med. Hyg. 96, 550–556 (2017).
Cizauskas, C. A. et al. Gastrointestinal helminths may affect host susceptibility to anthrax through seasonal immune trade-offs. BMC Ecol. 14, 27 (2014).
Havarua, Z., Turner, W. C. & Mfune, J. K. Seasonal variation in foraging behaviour of plains zebra (Equus quagga) may alter contact with the anthrax bacterium (Bacillus anthracis). Can. J. Zool. 92, 331–337 (2014).
Zidon, R., Garti, S., Getz, W. M. & Saltz, D. Zebra migration strategies and anthrax in Etosha National Park, Namibia. Ecosphere 8, e01925 (2017).
Schmidt, J. P. et al. Spatiotemporal fluctuations and triggers of ebola virus spillover. Emerg. Infect. Dis. 23, 415–422 (2017).
Kaul, R. B., Evans, M. V., Murdock, C. C. & Drake, J. M. Spatio-temporal spillover risk of yellow fever in Brazil. Parasites Vectors 11, 488 (2018).
Getz, W. M. et al. Making ecological models adequate. Ecol. Lett. 21, 153–166 (2018).
Blackburn, J. K. in Emerging and Endemic Pathogens (eds O'Connell, K., Skowronski, E., Sulakvelidze A. & Bakanidze, L.) 59–88 (Springer, 2010).
Walsh, M. G., de Smalen, A. W. & Mor, S. Climatic influence on the anthrax niche in warming northern latitudes. Sci. Rep. 8, 9269 (2018).
Hoffmann, C. et al. Persistent anthrax as a major driver of wildlife mortality in a tropical rainforest. Nature 548, 82–86 (2017).
Boria, R. A., Olson, L. E., Goodman, S. M. & Anderson, R. P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77 (2014).
Nsoesie, E. O. et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro Surveill. 21, 30234 (2016).
Barbet-Massin, M., Jiguet, F., Albert, C. H. & Thuiller, W. Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol. Evol. 3, 327–338 (2012).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Hengl, T. et al. SoilGrids250m: global gridded soil information based on machine learning. PloS ONE 12, e0169748 (2017).
Hay, S., Tatem, A., Graham, A., Goetz, S. & Rogers, D. Global environmental data for mapping infectious disease distribution. Adv. Parasitol. 62, 37–77 (2006).
Messina, J. P. et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans. R. Soc. Trop. Med. Hyg. 109, trv050 (2015).
Global Livestock Production Systems (Food and Agriculture Organization of the United Nations, 2011).
Robinson, T. P. et al. Mapping the global distribution of livestock. PLoS ONE 9, e96084 (2014).
Thornton, P. Mapping Poverty and Livestock in the Developing World (ILRI, 2002).
Ramesh, V., Gopalakrishna, T., Barve, S. & Melnick, D. J. IUCN greatly underestimates threat levels of endemic birds in the Western Ghats. Biol. Conserv. 210, 205–221 (2017).
Acknowledgements
The authors thank D. Pigott for helpful tips on BRT modelling; A. Barner for help obtaining World Animal Health Information System vaccination data; S. J. Ryan for general feedback and technical support; G. Simpson for visualization advice; P. Thornton for access to the global dataset of rural poor livestock keepers; T. A. Joyner for data support; and countless livestock and wildlife managers, clinicians and field technicians for contributing data points. Partial funding for this study was provided by NIH 1R01GM117617-01 to J.K.B. and W.M.G. C.J.C. was supported by the National Socio-Environmental Synthesis Center (SESYNC) under funding received from the National Science Foundation DBI-1639145. K.A. was supported in part under the National Science Foundation (NSF EEID grant 1518663). We also thank the Botswana Government Department of Wildlife and National Parks for their assistance and active collaboration on research directed at understanding Botswana anthrax dynamics.
Author information
Authors and Affiliations
Contributions
C.J.C., I.T.K. and J.K.B. conceived of the study. J.K.B., M.E.H.-J., I.T.K. and C.J.C. collected and georeferenced data. C.J.C., I.T.K. and J.K.B. designed the models, and C.J.C. ran models and analyses. N.R. contributed R code. All authors contributed to the writing and editing of the draft and approved the study before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Text and Discussion, Supplementary Figures 1–21, Supplementary Tables 1–33 and Supplementary References.
Rights and permissions
About this article
Cite this article
Carlson, C.J., Kracalik, I.T., Ross, N. et al. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat Microbiol 4, 1337–1343 (2019). https://doi.org/10.1038/s41564-019-0435-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0435-4
This article is cited by
-
Modelling climate change impacts on the spatial distribution of anthrax in Zimbabwe
BMC Public Health (2024)
-
Molecular characterization of an outbreak-involved Bacillus anthracis strain confirms the spillover of anthrax from West Africa
Infectious Diseases of Poverty (2024)
-
Combatting anthrax outbreaks across Nigeria’s national land borders: need to optimize surveillance with epidemiological surveys
Infectious Diseases of Poverty (2024)
-
Structure and function of the EA1 surface layer of Bacillus anthracis
Nature Communications (2023)
-
Investigation on an outbreak of cutaneous anthrax in a county of Shandong Province, China, 2021
BMC Infectious Diseases (2022)