Abstract
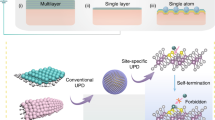
Developing active and stable atomically dispersed catalysts is challenging because of weak non-specific interactions between catalytically active metal atoms and supports. Here we demonstrate a general method for synthesizing atomically dispersed catalysts via photochemical defect tuning for controlling oxygen-vacancy dynamics, which can induce specific metal–support interactions. The developed synthesis method offers metal-dynamically stabilized atomic catalysts, and it can be applied to reducible metal oxides, including TiO2, ZnO and CeO2, containing various catalytically active transition metals, including Pt, Ir and Cu. The optimized Pt-DSA/TiO2 shows unprecedentedly high photocatalytic hydrogen evolution activity, producing 164 mmol g−1 h−1 with a turnover frequency of 1.27 s−1. Furthermore, it generates 42.2 mmol gsub−1 of hydrogen via a non-recyclable-plastic-photoreforming process, achieving a total conversion of 98%; this offers a promising solution for mitigating plastic waste and simultaneously producing valuable energy sources.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data that support the findings of this study are included in this Article. All other data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Zhou, P., Luo, M. & Guo, S. Optimizing the semiconductor–metal-single-atom interaction for photocatalytic reactivity. Nat. Rev. Chem. 6, 823–838 (2022).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
DeRita, L. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Cao, L. et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 565, 631–635 (2019).
Yang, H. B. et al. Atomically dispersed Ni (i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Xu, H. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5, 623–632 (2020).
Shan, J. et al. Integrating interactive noble metal single-atom catalysts into transition metal oxide lattices. J. Am. Chem. Soc. 144, 23214–23222 (2022).
Lee, B.-H. et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 18, 620–626 (2019).
Teng, Z. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4, 374–384 (2021).
Shan, J. et al. Metal-metal interactions in correlated single-atom catalysts. Sci. Adv. 8, eabo0762 (2022).
Han, B. et al. Strong metal–support interactions between Pt single atoms and TiO2. Angew. Chem. Int. Ed. 59, 11824–11829 (2020).
Wang, H. et al. High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu–TiO2 photocatalysts. Nat. Mater. 22, 619–626 (2023).
Zhang, X. et al. A stable low-temperature H2-production catalyst by crowding Pt on α-MoC. Nature 589, 396–401 (2021).
Li, X. et al. Functional CeOx nanoglues for robust atomically dispersed catalysts. Nature 611, 284–288 (2022).
Wu, Z.-Y. et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nat. Synth. 1, 658–667 (2022).
Zhang, Y. et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. Nat. Commun. 13, 58 (2022).
Lu, F. et al. Engineering platinum–oxygen dual catalytic sites via charge transfer towards highly efficient hydrogen evolution. Angew. Chem. 132, 17865–17871 (2020).
Chen, Y. et al. Engineering the atomic interface with single platinum atoms for enhanced photocatalytic hydrogen production. Angew. Chem. 132, 1311–1317 (2020).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013).
Daelman, N., Capdevila-Cortada, M. & López, N. Dynamic charge and oxidation state of Pt/CeO2 single-atom catalysts. Nat. Mater. 18, 1215–1221 (2019).
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Liu, K. et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts. Nat. Commun. 11, 1263 (2020).
Lee, B.-H. et al. Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O. Energy Environ. Sci. 15, 601–609 (2022).
Setvín, M. et al. Reaction of O2 with subsurface oxygen vacancies on TiO2 anatase (101). Science 341, 988–991 (2013).
Chen, X., Liu, L., Yu, P. Y. & Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011).
Selcuk, S., Zhao, X. & Selloni, A. Structural evolution of titanium dioxide during reduction in high-pressure hydrogen. Nat. Mater. 17, 923–928 (2018).
Howe, R. F. & Gratzel, M. EPR study of hydrated anatase under UV irradiation. J. Phys. Chem. 91, 3906–3909 (1987).
Strunk, J., Vining, W. C. & Bell, A. T. A study of oxygen vacancy formation and annihilation in submonolayer coverages of TiO2 dispersed on MCM-48. J. Phys. Chem. C 114, 16937–16945 (2010).
Peper, J. L., Vinyard, D. J., Brudvig, G. W. & Mayer, J. M. Slow equilibration between spectroscopically distinct trap states in reduced TiO2 nanoparticles. J. Am. Chem. Soc. 139, 2868–2871 (2017).
Macino, M. et al. Tuning of catalytic sites in Pt/TiO2 catalysts for the chemoselective hydrogenation of 3-nitrostyrene. Nat. Catal. 2, 873–881 (2019).
Hejazi, S. et al. On the controlled loading of single platinum atoms as a Co‐catalyst on TiO2 anatase for optimized photocatalytic H2 generation. Adv. Mater. 32, 1908505 (2020).
Wan, J. et al. Defect effects on TiO2 nanosheets: stabilizing single atomic site Au and promoting catalytic properties. Adv. Mater. 30, 1705369 (2018).
Drouilly, C. et al. ZnO oxygen vacancies formation and filling followed by in situ photoluminescence and in situ EPR. J. Phys. Chem. C 116, 21297–21307 (2012).
Kim, H. H., Lee, H., Kang, J. K. & Choi, W. K. Photoluminescence and electron paramagnetic resonance spectroscopy for revealing visible emission of ZnO quantum dots. Ann. Phys. 534, 2100382 (2022).
Wang, L. et al. Oxygen vacancy clusters essential for the catalytic activity of CeO2 nanocubes for o-xylene oxidation. Sci. Rep. 7, 12845 (2017).
Murugan, B. & Ramaswamy, A. Defect-site promoted surface reorganization in nanocrystalline ceria for the low-temperature activation of ethylbenzene. J. Am. Chem. Soc. 129, 3062–3063 (2007).
Ji, S. et al. Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020).
Uekert, T., Kuehnel, M. F., Wakerley, D. W. & Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 11, 2853–2857 (2018).
Lee, W. H. et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat. Nanotechnol. 18, 754–762 (2023).
Uekert, T., Pichler, C. M., Schubert, T. & Reisner, E. Solar-driven reforming of solid waste for a sustainable future. Nat. Sustain. 4, 383–391 (2021).
Zhang, S. et al. Boosted photoreforming of plastic waste via defect-rich NiPS3 nanosheets. J. Am. Chem. Soc. 145, 6410–6419 (2023).
Uekert, T., Kasap, H. & Reisner, E. Photoreforming of nonrecyclable plastic waste over a carbon nitride/nickel phosphide catalyst. J. Am. Chem. Soc. 141, 15201–15210 (2019).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Matsubara, M., Saniz, R., Partoens, B. & Lamoen, D. Doping anatase TiO2 with group V-b and VI-b transition metal atoms: a hybrid functional first-principles study. Phys. Chem. Chem. Phys. 19, 1945–1952 (2017).
Cargnello, M. et al. Engineering titania nanostructure to tune and improve its photocatalytic activity. Proc. Natl Acad. Sci. USA 113, 3966–3971 (2016).
Ou, G. et al. Tuning defects in oxides at room temperature by lithium reduction. Nat. Commun. 9, 1302 (2018).
Wei, T., Zhu, Y., Wu, Y., An, X. & Liu, L.-M. Effect of single-atom cocatalysts on the activity of faceted TiO2 photocatalysts. Langmuir 35, 391–397 (2018).
Acknowledgements
This research was supported by the Institute for Basic Science (IBS-R006-D1) and the National Supercomputing Center with supercomputing resources (KSC-2022-CRE-0146).
Author information
Authors and Affiliations
Contributions
C.W.L. and B.-H.L. conceived the idea and designed the research. C.W.L., B.-H.L. and S.P. designed the experiments. C.W.L. and S.P. conducted the photocatalytic measurements and analysis. J. Han and M.K. performed the DFT calculations and analysis. C.W.L., Y.J., J. Heo, K.L., W.K. and S.Y. conducted the data acquisition for material characterization and the subsequent analysis. C.W.L., B.-H.L., S.P. and M.K. wrote the original paper. M.S.B., J.R., K.T.N. and T.H. reviewed and edited the paper. T.H. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematic illustration of synthesizing Pt-DSA/TiO2 by photochemical defect tuning process.
A schematic illustration with photographs for the synthesis of Pt-DSA/TiO2 through the photochemical defect tuning process.
Extended Data Fig. 2 STEM and STEM-EDS elemental mapping images of Pt/TiO2 and Pt-NP/TiO2.
a, The Pt/TiO2 catalyst was produced through direct light irradiation on a mixture of Pt precursor (0.7 wt%) and anatase TiO2. In this particular case, Pt particles agglomerated severely, resulting in an irregular dispersion of Pt. b, The Pt-NP/TiO2 catalyst was prepared by physisorption of pre-made Pt nanoparticle on anatase TiO2.
Extended Data Fig. 3 Characterization of Pt-SA/hydrogenated TiO2.
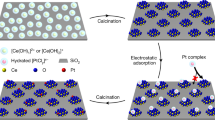
Pt deposition on hydrogenated TiO2, which is a conventional atomic metal deposition technique, was conducted and subsequently analyzed. a, EPR spectra of hydrogenated commercial anatase TiO2 and Pt-SA/hydrogenated TiO2. b, STEM and STEM-EDS elemental mapping images of Pt-SA/hydrogenated TiO2. c, XPS spectra of Pt-SA/hydrogenated TiO2. In (b) and (c), agglomeration of Pt was detected. d, Schematic illustration of the deposition of Pt metals on TiO2 through conventional wet-impregnation method on hydrogenated TiO2. The loading amount of Pt-SA/hydrogenated TiO2 was 0.1 wt%, with identical concentrations of the Pt precursor solution and stabilization time as the conditions used for Pt-DSA 0.7 wt%/TiO2.
Extended Data Fig. 4 EPR spectra of air regeneration process of irradiated ZnO and CeO2.
a, b, the EPR signal related to surface VOs vanished upon exposure to the air. This phenomenon suggests that photochemically induced surface VOs can naturally (a) revert back to bulk VOs for ZnO and (b) be refilled for CeO2, through the contact with oxygen in the air.
Extended Data Fig. 5 The pictures of synthesized M (Pt, Ir and Cu)-DSA/supports (TiO2, ZnO and CeO2) catalysts.
a, The pictures of pristine TiO2 (left) and Pt-DSA/TiO2 (right). b, c, d, The samples in the left, middle and right side represent Pt, Ir and Cu-DSA/ (b) TiO2, (c) ZnO and (d) CeO2, respectively.
Extended Data Fig. 6 XPS spectra and XRD patterns of Pt-DSA/TiO2, ZnO and CeO2.
XPS spectrum of Pt-DSA/TiO2 is provided in Fig. 2f. a, The Pt-DSA/TiO2 showed identical XRD patterns which corresponded with anatase TiO2 (JCPDS card 96-720-6076). b,c, XPS spectra of the Pt-DSA/ (b) ZnO (a pink line is Al 2p signal which comes from residual Al in commercial ZnO (Supplementary Fig. 1b)) and (c) CeO2. d,e, XRD patterns of Pt-DSA/ (d) ZnO and (e) CeO2 showed identical patterns with bare ZnO (JCPDS card 96-900-4182) and CeO2 (JCPDS card 96-900-9009), respectively.
Extended Data Fig. 7 XPS spectra and XRD patterns of M(Ir and Cu)-DSA/TiO2, ZnO and CeO2.
a, b, c, XPS spectra of the Ir-DSA/ (a) TiO2 (a pink line is Na 2 s signal which comes from residual Na in commercial anatase TiO2 (Supplementary Fig. 1c)), (b) ZnO and (c) CeO2. The resulting Ir peak occurred between the position of Ir4+ and Ir0. d,e,f, XPS spectra of the Cu-DSA/ (d) TiO2, (e) ZnO and (f) CeO2 (a pink line is signal which comes from residual elements in commercial CeO2 (Supplementary Fig. 1d)). g,h,i, XRD patterns of Ir and Cu-DSA/ (g) TiO2, (h) ZnO and (i) CeO2 showed identical patterns with bare TiO2 (JCPDS card 96-720-6076), ZnO (JCPDS card 96-900-4182) and CeO2 (JCPDS card 96-900-9009), respectively.
Extended Data Fig. 8 Quantification of the consumed reactants before and after the photo-reforming of PET through the analysis of 1H NMR spectra.
The amount of EG and TPA consumed after 40 h of the reaction in Fig. 5e was calculated using the calibration curves in (b) and (d). The calculated amounts of EG and TPA before the reaction were 111 μmol and 20.6 μmol, respectively. After 40 h of the reaction, 100% of EG (111 μmol) and 91% of TPA (18.7 μmol) were consumed. a,b,c,d, The calibration curves for (b) EG and (d) TPA were obtained based on 1H NMR data from different concentrations of (a) EG and (c) TPA, respectively. The normalized areas of EG and TPA against DMSO (internal standard) were plotted depending on concentrations, and linear regression equations were obtained. TPA was detected as a form of terephthalate in the 1H NMR (D2O condition) data.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, C.W., Lee, BH., Park, S. et al. Photochemical tuning of dynamic defects for high-performance atomically dispersed catalysts. Nat. Mater. 23, 552–559 (2024). https://doi.org/10.1038/s41563-024-01799-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-024-01799-y