Abstract
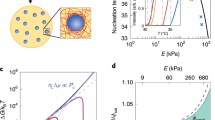
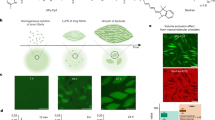
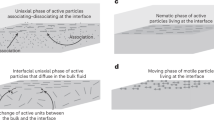
Demixing binary liquids is a ubiquitous transition explained using a well-established thermodynamic formalism that requires the equality of intensive thermodynamics parameters across phase boundaries. Demixing transitions also occur when binary fluid mixtures are driven away from equilibrium, but predicting and designing such out-of-equilibrium transitions remains a challenge. Here we study the liquid–liquid phase separation of attractive DNA nanostars driven away from equilibrium using a microtubule-based active fluid. We find that activity lowers the critical temperature and narrows the range of coexistence concentrations, but only in the presence of mechanical bonds between the liquid droplets and reconfiguring active fluid. Similar behaviours are observed in numerical simulations, suggesting that the activity suppression of the critical point is a generic feature of active liquid–liquid phase separation. Our work describes a versatile platform for building soft active materials with feedback control and providing an insight into self-organization in cell biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
Code availability
The numerical code used to generate the thermotical phase diagram is available via GitHub at https://github.com/fcaballerop/nematicPhaseFieldFoam.
References
Wolde, P. R. T. & Frenkel, D. Enhancement of protein crystal nucleation by critical density fluctuations. Science 277, 1975–1978 (1997).
van de Witte, P., Dijkstra, P., Van den Berg, J. & Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 117, 1–31 (1996).
Ianiro, A. et al. Liquid–liquid phase separation during amphiphilic self-assembly. Nat. Chem. 11, 320–328 (2019).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Hinze, J. O. Fundamentals of the hydrodynamic mechanism of splitting in dispersion processes. AlChE J. 1, 289–295 (1955).
Onuki, A. Phase transitions of fluids in shear flow. J. Phys. Condens. Matter 9, 6119 (1997).
Berthier, L. Phase separation in a homogeneous shear flow: morphology, growth laws, and dynamic scaling. Phys. Rev. E 63, 051503 (2001).
Han, C. C., Yao, Y., Zhang, R. & Hobbie, E. K. Effect of shear flow on multi-component polymer mixtures. Polymer 47, 3271–3286 (2006).
Olmsted, P. D. Perspectives on shear banding in complex fluids. Rheol. Acta 47, 283–300 (2008).
Zhang, R. et al. Phase separation mechanism of polybutadiene/polyisoprene blends under oscillatory shear flow. Macromolecules 41, 6818–6829 (2008).
Pine, D., Easwar, N., Maher, J. V. & Goldburg, W. Turbulent suppression of spinodal decomposition. Phys. Rev. A 29, 308 (1984).
Marchetti, M. C. et al. Hydrodynamics of soft active matter. Rev. Mod. Phys. 85, 1143 (2013).
Nakashima, K. K., van Haren, M. H., André, A. A., Robu, I. & Spruijt, E. Active coacervate droplets are protocells that grow and resist Ostwald ripening. Nat. Commun. 12, 3819 (2021).
Nguyen, N. H., Klotsa, D., Engel, M. & Glotzer, S. C. Emergent collective phenomena in a mixture of hard shapes through active rotation. Phys. Rev. Lett. 112, 075701 (2014).
Soni, V. et al. The odd free surface flows of a colloidal chiral fluid. Nat. Phys. 15, 1188–1194 (2019).
Testa, A. et al. Sustained enzymatic activity and flow in crowded protein droplets. Nat. Commun. 12, 6293 (2021).
Adkins, R. et al. Dynamics of active liquid interfaces. Science 377, 768–772 (2022).
Theurkauff, I., Cottin-Bizonne, C., Palacci, J., Ybert, C. & Bocquet, L. Dynamic clustering in active colloidal suspensions with chemical signaling. Phys. Rev. Lett. 108, 268303 (2012).
Palacci, J., Sacanna, S., Steinberg, A. P., Pine, D. J. & Chaikin, P. M. Living crystals of light-activated colloidal surfers. Science 339, 936–940 (2013).
Redner, G. S., Hagan, M. F. & Baskaran, A. Structure and dynamics of a phase-separating active colloidal fluid. Phys. Rev. Lett. 110, 055701 (2013).
Zhang, J., Alert, R., Yan, J., Wingreen, N. S. & Granick, S. Active phase separation by turning towards regions of higher density. Nat. Phys. 17, 961–967 (2021).
Fily, Y. & Marchetti, M. C. Athermal phase separation of self-propelled particles with no alignment. Phys. Rev. Lett. 108, 235702 (2012)
Redner, G. S., Baskaran, A. & Hagan, M. F. Reentrant phase behavior in active colloids with attraction. Phys. Rev. E 88, 012305 (2013).
Schwarz-Linek, J. et al. Phase separation and rotor self-assembly in active particle suspensions. Proc. Natl Acad. Sci. USA 109, 4052–4057 (2012).
Ross, T. D. et al. Controlling organization and forces in active matter through optically defined boundaries. Nature 572, 224–229 (2019).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Biffi, S. et al. Phase behavior and critical activated dynamics of limited-valence DNA nanostars. Proc. Natl Acad. Sci. USA 110, 15633–15637 (2013).
Sanchez, T., Chen, D. T., DeCamp, S. J., Heymann, M. & Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 491, 431–434 (2012).
Conrad, N., Kennedy, T., Fygenson, D. K. & Saleh, O. A. Increasing valence pushes DNA nanostar networks to the isostatic point. Proc. Natl Acad. Sci. USA 116, 7238–7243 (2019).
Jeon, B.-j et al. Salt-dependent properties of a coacervate-like, self-assembled DNA liquid. Soft Matter 14, 7009–7015 (2018).
Nguyen, D. T., Jeon, B.-j, Abraham, G. R. & Saleh, O. A. Length-dependence and spatial structure of DNA partitioning into a DNA liquid. Langmuir 35, 14849–14854 (2019).
Tayar, A. M., Hagan, M. F. & Dogic, Z. Active liquid crystals powered by force-sensing DNA-motor clusters. Proc. Natl Acad. Sci. USA 118, e2102873118 (2021).
Hilitski, F. et al. Measuring cohesion between macromolecular filaments one pair at a time: depletion-induced microtubule bundling. Phys. Rev. Lett. 114, 138102 (2015).
Andre, A. A. M., Yewdall, N. A. & Spruijt, E. Crowding-induced phase separation and gelling by co-condensation of PEG in NPM1-rRNA condensates. Biophys. J. 122, 397–407 (2023).
Bate, T. E., Jarvis, E. J., Varney, M. E. & Wu, K.-T. Collective dynamics of microtubule-based 3D active fluids from single microtubules. Soft Matter 15, 5006–5016 (2019).
Singh, R. & Cates, M. Hydrodynamically interrupted droplet growth in scalar active matter. Phys. Rev. Lett. 123, 148005 (2019).
Tiribocchi, A., Wittkowski, R., Marenduzzo, D. & Cates, M. E. Active model H: scalar active matter in a momentum-conserving fluid. Phys. Rev. Lett. 115, 188302 (2015)
Chandrakar, P. et al. Confinement controls the bend instability of three-dimensional active liquid crystals. Phys. Rev. Lett. 125, 257801 (2020).
Thampi, S. P., Golestanian, R. & Yeomans, J. M. Velocity correlations in an active nematic. Phys. Rev. Lett. 111, 118101 (2013).
Blow, M. L., Thampi, S. P. & Yeomans, J. M. Biphasic, lyotropic, active nematics. Phys. Rev. Lett. 113, 248303 (2014).
Giomi, L. & DeSimone, A. Spontaneous division and motility in active nematic droplets. Phys. Rev. Lett. 112, 147802 (2014).
Hughes, R. & Yeomans, J. M. Collective chemotaxis of active nematic droplets. Phys. Rev. E 102, 020601 (2020).
Marenduzzo, D., Orlandini, E., Cates, M. & Yeomans, J. Steady-state hydrodynamic instabilities of active liquid crystals: hybrid lattice Boltzmann simulations. Phys. Rev. E 76, 031921 (2007).
Simha, R. A. & Ramaswamy, S. Hydrodynamic fluctuations and instabilities in ordered suspensions of self-propelled particles. Phys. Rev. Lett. 89, 058101 (2002).
Cates, M. E. in Active Matter and Nonequilibrium Statistical Physics: Lecture Notes of the Les Houches Summer School Vol. 112 (eds Gompper, G., Cristina Marchetti, M., Tailleur, J. & Yeomans, J. M.) (Oxford Univ. Press, 2019).
Foster, P. J. et al. Dissipation and energy propagation across scales in an active cytoskeletal material. Proc. Natl Acad. Sci. USA 120, e2207662120 (2023).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017)
Fritsch, A. W. et al. Local thermodynamics govern formation and dissolution of Caenorhabditis elegans P granule condensates. Proc. Natl Acad. Sci. USA 118, e2102772118 (2021).
Setru, S. U. et al. A hydrodynamic instability drives protein droplet formation on microtubules to nucleate branches. Nat. Phys. 17, 493-498 (2021).
Wiegand, T. & Hyman, A. A. Drops and fibers—how biomolecular condensates and cytoskeletal filaments influence each other. Emerg. Top. Life Sci. 4, 247–261 (2020).
Castoldi, M. & Popova, A. V. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr. Purif. 32, 83–88 (2003).
Tayar, A. M., Lemma, L. M. & Dogic, Z. Assembling microtubule-based active matter. Methods Mol. Biol. 2430, 151–183 (2022).
Young, E. C., Berliner, E., Mahtani, H. K., Perezramirez, B. & Gelles, J. Subunit interactions in dimeric kinesin heavy-chain derivated that lack the kinesin rod. J. Biol. Chem. 270, 3926–3931 (1995).
Derr, N. D. et al. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. Science 338, 662–665 (2012).
Goodman, B. S. & Reck-Peterson, S. L. in Reconstituting the Cytoskeleton Methods in Enzymology (ed Vale, R. D.) Vol. 540, 169–188 (2014).
Mitchell, N. P. & Cislo, D. J. TubULAR: tracking in toto deformations of dynamic tissues via constrained maps. Preprint at bioRxiv https://doi.org/10.1101/2022.04.19.488840 (2022).
Acknowledgements
We thank P. Gulati, Z. You, G. Abraham and S. Takatori for fruitful discussions. We thank N. Mitchell for help with the analysis of the 3D images. This work was primarily supported by the US Department of Energy, Basic Energy Sciences, through award DE-SC001973. The initial stages of the theoretical modelling were supported by NSF-DMR-2041459. We also acknowledge the use of the Brandeis MRSEC Bio-Synthesis Facility, which is supported by NSF-MRSEC-2011846. O.A.S. acknowledges support from the W.M. Keck Foundation. A.M.T. is a Simons Foundation Fellow of the Life Sciences Research Foundation and is an Awardee of the Weizmann Institute of Science–National Postdoctoral Award Program for Advancing Women in Science.
Author information
Authors and Affiliations
Contributions
A.M.T., O.A.S. and Z.D. designed the experiments. A.M.T. performed the experimental work and data analysis. T.A. helped with the data analysis. F.C. and M.C.M. developed the theory. A.M.T., M.C.M. and Z.D. wrote the manuscript. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Steve Granick for his contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, captions for Videos 1–7 and theoretical model.
Supplementary Video 1
Interaction of DNA nanostar droplets and active fluid. Part 1: DNA droplet with no kinesin–DNA. The droplet is advected by MT flows using traditional streptavidin–biotin motor clusters. Overlaid video of DNA droplet (cyan), embedded in an MT active gel (red). Part 2: dense droplets with no kinesin–DNA are advected and deformed by the reconfigurable MT network powered by kinesin–DNA nanostars in the dilute phase. The video is compatible with the data shown in Fig. 1c. Temperature is 19 °C; kinesin–DNA concentration is 2.5 μM; scale bar, 50 μm. The time stamp on the videos indicates the beginning of acquisition.
Supplementary Video 2
LLPS in passive and active samples during temperature ramp-up. Equilibrium LLPS containing DNA nanostars but no active fluid (left). Data with kinesin–DNA in an active MT gel (right). Kinesin concentration, 2.5 µM. The temperature is indicated on the side panel. Each temperature step lasts for 45 min. Scale bar, 100 µm. The time stamp on the videos indicates the beginning of acquisition.
Supplementary Video 3
Dynamics of LLPS with kinesin–DNA nanostars during ATP depletion. Kinesin–DNA depletes the available ATP, leading to a decrease in the active flow speeds. During the slow down, new DNA droplets form. To visualize the formation of new droplets, the contrast was enhanced, which saturated the large droplets. Videos are taken at T = 19 °C and kinesin–DNA concentration of 3.5 μM. The time stamp on the videos indicates the beginning of acquisition.
Supplementary Video 4
Interactions of a droplet with an active fluid in absence of kinesin–DNA. MT–droplet interactions on the droplet surface in a system lacking kinesin–DNA. The droplet is advected by MT flows using traditional streptavidin–biotin motor clusters. The projection layer of the MTs on the surface of the DNA droplet is 11 μm from the surface. Parts 1–3 show the visualization of MT flows on three different droplets. Temperature is 17 °C, the active fluid speed is between 0.3 and 1.0 μm s–1 and the scale is noted by the axis. The time stamp on the videos indicates the beginning of acquisition.
Supplementary Video 5
Interactions of a droplet with an active fluid in the presence of kinesin–DNA. The visualization of MT–droplet interactions in a kinesin–DNA droplet. The droplet is advected, deformed and broken by MT flows powered by kinesin–DNA in the dilute phase. The droplets align with the long axis of the MT bundles on the interface. Parts 1–3 show the visualization of MT flows on three different droplets. The projection layer of MTs on the surface of the DNA droplet is 5.5 μm from the surface. The videos are complementary to the data shown in Fig. 5b. The temperature is 17.5 °C, the external fluid velocity is 0.25 μm s–1, kinesin concentration is 3.5 μM and the scale bar is noted by the axis. The time stamp on the videos indicates the beginning of acquisition.
Supplementary Video 6
Interfacial velocity profile. Two-dimensional confocal image of beads embedded in a DNA droplet. The droplets are faintly labelled with a YOYO dye. The 200 µm beads are larger than the nanostar’s core-to-core distances, indicating local deformations. Temperature is 17.5 °C; scale bar, 100 μm. Part 1: DNA droplet with no kinesin–DNA. The droplet is advected by active flows, which are generated by streptavidin–kinesin clusters. External fluid velocity is 1 μm s–1. Part 2: droplets in a system with kinesin–DNA nanostars. The droplet is advected and deformed by active flows. Kinesin concentration, 3.5 μM The time stamp on the videos indicates the beginning of acquisition.
Supplementary Video 7
Interfacial flow profile in numerical simulations. Numerical integration inside and outside the coexistence region (using the equation mentioned in the main text). We change activity α between the two runs to cross the coexistence region. Inside the coexistence region: low activity, α = −10 (left), we observe the arrested phase separation described in the main text, in which a fully phase-separated initial state breaks down until it reaches a steady state of very dynamic finite-sized droplets; high activity, α = −50 (right), active stresses are strong enough to stabilize the uniform state, and the system is stirred by the nematic, fully mixing both species. The parameters are aQ = −aϕ = bQ = bϕ = γ = λ = η = 1, K = 5 × 10−6, M = 0.1, k = 0.004. The integration is done on a square lattice of 128 × 128, with a lattice spacing of Δx = 0.1 and a time step of Δt = 0.001.
Source data
Source Data Fig. 2
Numerical source data for Fig. 2.
Source Data Fig. 3
Numerical source data for Fig. 3.
Source Data Fig. 4
Numerical source data for Fig. 4.
Source Data Fig. 5
Numerical source data for Fig. 5.
Source Data Fig. 6
Numerical source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tayar, A.M., Caballero, F., Anderberg, T. et al. Controlling liquid–liquid phase behaviour with an active fluid. Nat. Mater. 22, 1401–1408 (2023). https://doi.org/10.1038/s41563-023-01660-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01660-8
This article is cited by
-
Self-assembly of stabilized droplets from liquid–liquid phase separation for higher-order structures and functions
Communications Chemistry (2024)
-
TubULAR: tracking in toto deformations of dynamic tissues via constrained maps
Nature Methods (2023)
-
Seriously non-thermal thermodynamics
Nature Materials (2023)