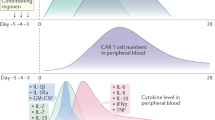
Abstract
Chimeric antigen receptor T (CAR T) cell immunotherapy is successful at treating many cancers. However, it often induces life-threatening cytokine release syndrome (CRS) and neurotoxicity. Here, we show that in situ conjugation of polyethylene glycol (PEG) to the surface of CAR T cells (‘PEGylation’) creates a polymeric spacer that blocks cell-to-cell interactions between CAR T cells, tumour cells and monocytes. Such blockage hinders intensive tumour lysing and monocyte activation by CAR T cells and, consequently, decreases the secretion of toxic cytokines and alleviates CRS-related symptoms. Over time, the slow expansion of CAR T cells decreases PEG surface density and restores CAR T cell–tumour-cell interactions to induce potent tumour killing. This occurs before the restoration of CAR T cell–monocyte interactions, opening a therapeutic window for tumour killing by CAR T cells before monocyte overactivation. Lethal neurotoxicity is also lower when compared with treatment with the therapeutic antibody tocilizumab, demonstrating that in situ PEGylation of CAR T cells provides a materials-based strategy for safer cellular immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper.
References
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
MacKay, M. et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat. Biotechnol. 38, 233–244 (2020).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Mikkilineni, L. & Kochenderfer, J. N. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 18, 71–84 (2021).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Brudno, J. N. et al. T cells genetically modified to express an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 36, 2267 (2018).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 1–14 (2018).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 1–11 (2021).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Cao, J.-X. et al. The incidence of cytokine release syndrome and neurotoxicity of CD19 chimeric antigen receptor–T cell therapy in the patient with acute lymphoblastic leukemia and lymphoma. Cytotherapy 22, 214–226 (2020).
Gauthier, J. & Turtle, C. J. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr. Res. Transl. Med. 66, 50–52 (2018).
Bonifant, C. L., Jackson, H. J., Brentjens, R. J. & Curran, K. J. Toxicity and management in CAR T-cell therapy. Mol. Ther.-Oncolytics 3, 16011 (2016).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra225 (2014).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy.Nat. Rev. Immunol. 22, 85–96 (2021).
Majzner, R. G. & Mackall, C. L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25, 1341–1355 (2019).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Turtle, C. J. et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138 (2016).
Giavridis, T. et al. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Sachdeva, M., Duchateau, P., Depil, S., Poirot, L. & Valton, J. Granulocyte–macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J. Biol. Chem. 294, 5430–5437 (2019).
Frigault, M. J. et al. A phase II trial of anakinra for the prevention of CAR-T cell mediated neurotoxicity. Blood 138, 2814 (2021).
Wei, J. et al. The model of cytokine release syndrome in CAR T-cell treatment for B-cell non-Hodgkin lymphoma. Signal. Transduct. Target. Ther. 5, 1–9 (2020).
Wagner, D. H. Jr, Stout, R. D. & Suttles, J. Role of the CD40‐CD40 ligand interaction in CD4+ T cell contact‐dependent activation of monocyte interleukin‐1 synthesis. Eur. J. Immunol. 24, 3148–3154 (1994).
Nashleanas, M. & Scott, P. Activated T cells induce macrophages to produce NO and control Leishmania major in the absence of tumor necrosis factor receptor p55. Infect. Immun. 68, 1428–1434 (2000).
McInnes, I. B., Leung, B. P., Sturrock, R. D., Field, M. & Liew, F. Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat. Med. 3, 189–195 (1997).
Avice, M.-N., Sarfati, M., Triebel, F., Delespesse, G. & Demeure, C. E. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-α and IL-12 production by monocytes and dendritic cells. J. Immunol. 162, 2748–2753 (1999).
Birkland, T. P., Sypek, J. P. & Wyler, D. J. Soluble TNF and membrane TNF expressed on CD4+ T lymphocytes differ in their ability to activate macrophage antileishmanial defense. J. Leukoc. Biol. 51, 296–299 (1992).
Parry, S. L., Sebbag, M., Feldmann, M. & Brennan, F. M. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-alpha. J. Immunol. 158, 3673–3681 (1997).
Bird, L. Calming the cytokine storm. Nat. Rev. Immunol. 18, 417–417 (2018).
Rooney, C. & Sauer, T. Modeling cytokine release syndrome. Nat. Med. 24, 705–706 (2018).
Kolate, A. et al. PEG—a versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 192, 67–81 (2014).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Spiciarich, D. R. et al. Bioorthogonal labeling of human prostate cancer tissue slice cultures for glycoproteomics. Angew. Chem. Int. Ed. 56, 8992–8997 (2017).
Prescher, J. A., Dube, D. H. & Bertozzi, C. R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Guo, C. et al. Bio-orthogonal conjugation and enzymatically triggered release of proteins within multi-layered hydrogels. Acta Biomater. 56, 80–90 (2017).
Nagahama, K., Kimura, Y. & Takemoto, A. Living functional hydrogels generated by bioorthogonal cross-linking reactions of azide-modified cells with alkyne-modified polymers. Nat. Commun. 9, 1–11 (2018).
Wang, H. et al. Metabolic labeling and targeted modulation of dendritic cells. Nat. Mater. 19, 1244–1252 (2020).
Mehvar, R. Modulation of the pharmacokinetics and pharmacodynamics of proteins by polyethylene glycol conjugation.J. Pharm. Pharm. Sci. 3, 125–136 (2000).
Patel, K. et al. Use of the IL-6R antagonist tocilizumab in hospitalized COVID-19 patients.J. Intern. Med. 289, 430–433 (2020).
Hay, K. A. Cytokine release syndrome and neurotoxicity after CD 19 chimeric antigen receptor‐modified (CAR‐) T cell therapy. Br. J. Haematol. 183, 364–374 (2018).
Nair, L. S. & Laurencin, C. T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32, 762–798 (2007).
Acharya, G. et al. The hydrogel template method for fabrication of homogeneous nano/microparticles. J. Control. Release 141, 314–319 (2010).
Mitchell, G. & Miller, J. Cell to cell interaction in the immune response: II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J. Exp. Med. 128, 821–837 (1968).
Acknowledgements
M.J.M. acknowledges support from an NIH Director’s New Innovator Award (no. DP2TR002776), an NSF CAREER Award (no. CBET-2145491), a Burroughs Wellcome Fund Career Award at the Scientific Interface and the American Cancer Society (no. RSG-22-122-01-ET). The authors thank the June lab for the help on CAR T cell preparation. The authors also acknowledge the NIH S10 grant (1S10OD026986) for the support on a cell sorter.
Author information
Authors and Affiliations
Contributions
N.G. and M.J.M. conceived and designed the experiments. N.G., X.H., L.X. and A.G.H. performed the experiments. N.G., L.X., X.H. and R.E.M. analysed the data. N.G. and M.J.M. wrote the manuscript. M.M.B. and A.E.M. edited the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.G. and M.J.M. have filed a patent application related to this study. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Bruno De Geest, Christopher Jewell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of the CAR T cell ex vivo PEGylation strategy with the in situ PEGylation strategy.
a, Raji tumour-bearing mouse model was constructed, and mice were treated with either ex vivo PEGylated CAR T cells or regular CAR T-azide cells at day 0. On day 1, the mice receiving regular CAR T-azide cells developed high fever (ΔT > 2 °C), after which DBCO-PEG600k (in situ PEGylation) was injected. Mouse body temperature (b), tumour burden (c), blood IL-6 levels (d) and CAR T cell levels (e) were monitored. f, quantification of tumour burden in different groups at day 35. g, Kaplan–Meyer survival plots. Data in (b), (d), (e), and (f) are shown as mean ± s.d. (n = 5). Statistical differences in (b), (d), and (e) were calculated using two-way ANOVA with Tukey’s post hoc test. Statistical differences in (f) were calculated using one-way ANOVA with Tukey’s post hoc test. P values indicated in the figure are from the comparisons on day 7. Statistical differences in (g) were conducted using a Mantel–Cox two-sided log-rank test (n = 5). P values are indicated.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, N., Han, X., Xue, L. et al. In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity. Nat. Mater. 22, 1571–1580 (2023). https://doi.org/10.1038/s41563-023-01646-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01646-6
This article is cited by
-
Novel insights into TCR-T cell therapy in solid neoplasms: optimizing adoptive immunotherapy
Experimental Hematology & Oncology (2024)
-
Lyophilized lymph nodes for improved delivery of chimeric antigen receptor T cells
Nature Materials (2024)
-
Taming CAR T cell therapy toxicity
Nature Materials (2023)