Abstract
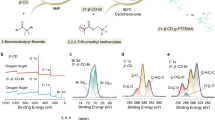
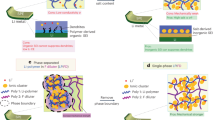
The solid–electrolyte interphase (SEI) is pivotal in stabilizing lithium metal anodes for rechargeable batteries. However, the SEI is constantly reforming and consuming electrolyte with cycling. The rational design of a stable SEI is plagued by the failure to control its structure and stability. Here we report a molecular-level SEI design using a reactive polymer composite, which effectively suppresses electrolyte consumption in the formation and maintenance of the SEI. The SEI layer consists of a polymeric lithium salt, lithium fluoride nanoparticles and graphene oxide sheets, as evidenced by cryo-transmission electron microscopy, atomic force microscopy and surface-sensitive spectroscopies. This structure is different from that of a conventional electrolyte-derived SEI and has excellent passivation properties, homogeneity and mechanical strength. The use of the polymer–inorganic SEI enables high-efficiency Li deposition and stable cycling of 4 V Li|LiNi0.5Co0.2Mn0.3O2 cells under lean electrolyte, limited Li excess and high capacity conditions. The same approach was also applied to design stable SEI layers for sodium and zinc anodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Goodenough, J. B. & Park, K.-S. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Choi, N. S. et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 51, 9994–10024 (2012).
Kim, H. et al. Metallic anodes for next generation secondary batteries. Chem. Soc. Rev. 42, 9011–9034 (2013).
Peled, E. & Menkin, S. Review—SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Cheng, X. B., Zhang, R., Zhao, C. Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Tikekar, M. D., Choudhury, S., Tu, Z. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016).
Sacci, R. L. et al. Direct visualization of initial SEI morphology and growth kinetics during lithium deposition by in situ electrochemical transmission electron microscopy. Chem. Commun. 50, 2104 (2014).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Ye, H. et al. Stable Li plating/stripping electrochemistry realized by a hybrid Li reservoir in spherical carbon granules with 3D conducting skeletons. J. Am. Chem. Soc. 139, 5916–5922 (2017).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016).
Li, G. et al. Stable metal battery anodes enabled by polyethylenimine sponge hosts by way of electrokinetic effects. Nat. Energy 3, 1076–1083 (2018).
Tung, S.-O., Ho, S., Yang, M., Zhang, R. & Kotov, N. A. A dendrite-suppressing composite ion conductor from aramid nanofibres. Nat. Commun. 6, 6152 (2015).
Cheng, X.-B. et al. Nanodiamonds suppress the growth of lithium dendrites. Nat. Commun. 8, 336 (2017).
Li, N.-W., Yin, Y.-X., Yang, C.-P. & Guo, Y.-G. An artificial solid electrolyte interphase layer for stable lithium metal anodes. Adv. Mater. 28, 1853–1858 (2016).
Dudney, N. J. Addition of a thin-film inorganic solid electrolyte (Lipon) as a protective film in lithium batteries with a liquid electrolyte. J. Power Sources 89, 176–179 (2000).
Kazyak, E., Wood, K. N. & Dasgupta, N. P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 27, 6457–6462 (2015).
Zhao, J. et al. Surface fluorination of reactive battery anode materials for enhanced stability. J. Am. Chem. Soc. 139, 11550–11558 (2017).
Liang, X. et al. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 6, 17119 (2017).
Tu, Z. et al. Designing artificial solid–electrolyte interphases for single-ion and high-efficiency transport in batteries. Joule 1, 394–406 (2017).
Liu, K. et al. Lithium metal anodes with an adaptive “Solid–liquid” interfacial protective layer. J. Am. Chem. Soc. 139, 4815–4820 (2017).
Choi, S. M. et al. Cycling characteristics of lithium metal batteries assembled with a surface modified lithium electrode. J. Power Sources 244, 363–368 (2013).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Suo, L., Hu, Y.-S., Li, H., Armand, M. & Chen, L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 4, 1481 (2013).
Basile, A., Bhatt, A. I. & O’Mullane, A. P. Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 7, ncomms11794 (2016).
Lu, Y., Korf, K., Kambe, Y., Tu, Z. & Archer, L. A. Ionic–liquid–nanoparticle hybrid electrolytes: applications in lithium metal batteries. Angew. Chem. Int. Ed. 53, 488–492 (2014).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Markevich, E., Salitra, G. & Aurbach, D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries. ACS Energy Lett. 2, 1337–1345 (2017).
Zhang, Y. et al. Dendrite-free lithium deposition with self-aligned nanorod structure. Nano Lett. 14, 6889–6896 (2014).
Chen, S. et al. Functional organosulfide electrolyte promotes an alternate reaction pathway to achieve high performance in lithium-sulfur batteries. Angew. Chem. Int. Ed. 55, 4231–4235 (2016).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Li, G. et al. Organosulfide-plasticized solid–electrolyte interphase layer enables stable lithium metal anodes for long-cycle lithium-sulfur batteries. Nat. Commun. 8, 850 (2017).
Zhang, H. et al. Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: progress and perspectives. Angew. Chem. Int. Ed. 57, 15002–15027 (2018).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 148, 405–416 (2002).
Gao, Y. et al. Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 139, 15288–15291 (2017).
Gao, Y. et al. Salt-based organic–inorganic nanocomposites: towards a stable lithium metal/Li10GeP2S12 solid electrolyte interface. Angew. Chem. Int. Ed. 57, 13608–13612 (2018).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Wang, X. et al. New insights on the structure of electrochemically deposited lithium metal and its solid electrolyte interphases via cryogenic TEM. Nano Lett. 17, 7606–7612 (2017).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
Foroozan, T. et al. Synergistic effect of graphene oxide for impeding the dendritic plating of Li. Adv. Funct. Mater. 28, 1705917 (2018).
Kovtyukhova, N. I. et al. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 11, 771–778 (1999).
Green, C. P. & Sader, J. E. Frequency response of cantilever beams immersed in viscous fluids near a solid surface with applications to the atomic force microscope. J. Appl. Phys. 98, 114913 (2005).
Kuznetsov, V. et al. Wet nanoindentation of the solid electrolyte interphase on thin film Si electrodes. ACS Appl. Mater. Interfaces 7, 23554–23563 (2015).
Greaves, G. N., Greer, A. L., Lakes, R. S. & Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 10, 823–837 (2011).
Carpick, R. W., Ogletree, D. F. & Salmeron, M. A General equation for fitting contact area and friction vs load measurements. J Colloid Interface Sci. 400, 395–400 (1999).
Piétrement, O. & Troyon, M. General equations describing elastic indentation depth and normal contact stiffness versus load. J. Colloid Interface Sci. 226, 166–171 (2000).
Ebenstein, D. M. & Wahl, K. J. A comparison of JKR-based methods to analyze quasi-static and dynamic indentation force curves. J. Colloid Interface Sci. 298, 652–662 (2006).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy, through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) award no. DE-EE0008198. Z.Y., Y.C.L. and T.E.M. acknowledge support from the National Science Foundation under grant DMR-1807116. X.H. and S.H.K. acknowledge support from the National Science Foundation under grant CMMI-1435766.
Author information
Authors and Affiliations
Contributions
Y.G., T.E.M. and Do.W. conceived the idea, Y.G. and Do.W. designed the experiments, and Do.W. directed the project. Y.G. performed the material preparation and chemical and morphological characterization. Z.Y. prepared the graphene oxide materials. H.W. prepared the samples for cryo-TEM experiments. J.L.G. performed the cryo-TEM experiments. Y.G. and T.C. performed the battery tests. X.H. conducted the AFM indentation test. Y.G. and Y.C.L. performed the electrochemical impedance spectroscopy test. Y.G. and Da.W. conducted the SEM test. All authors discussed and analysed the data. Y.G., S.H.K, T.E.M. and Do.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–42, Supplementary Table 1, Supplementary References 1–9
Rights and permissions
About this article
Cite this article
Gao, Y., Yan, Z., Gray, J.L. et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019). https://doi.org/10.1038/s41563-019-0305-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0305-8
This article is cited by
-
Ultrafast laser one-step construction of 3D micro-/nanostructures achieving high-performance zinc metal anodes
PhotoniX (2024)
-
Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries
Nature Energy (2024)
-
Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries
Nature Communications (2024)
-
Enhancing lithium-metal battery longevity through minimized coordinating diluent
Nature Energy (2024)
-
Standardized cycle life assessment of batteries using extremely lean electrolytic testing conditions
Communications Materials (2024)