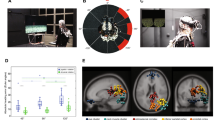
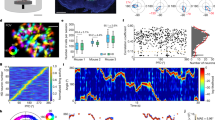
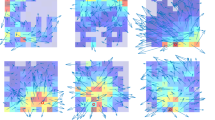
Abstract
Information about heading direction is critical for navigation as it provides the means to orient ourselves in space. However, given that veridical head-direction signals require physical rotation of the head and most human neuroimaging experiments depend upon fixing the head in position, little is known about how the human brain is tuned to such heading signals. Here we adress this by asking 52 healthy participants undergoing simultaneous electroencephalography and motion tracking recordings (split into two experiments) and 10 patients undergoing simultaneous intracranial electroencephalography and motion tracking recordings to complete a series of orientation tasks in which they made physical head rotations to target positions. We then used a series of forward encoding models and linear mixed-effects models to isolate electrophysiological activity that was specifically tuned to heading direction. We identified a robust posterior central signature that predicts changes in veridical head orientation after regressing out confounds including sensory input and muscular activity. Both source localization and intracranial analysis implicated the medial temporal lobe as the origin of this effect. Subsequent analyses disentangled head-direction signatures from signals relating to head rotation and those reflecting location-specific effects. Lastly, when directly comparing head direction and eye-gaze-related tuning, we found that the brain maintains both codes while actively navigating, with stronger tuning to head direction in the medial temporal lobe. Together, these results reveal a taxonomy of population-level head-direction signals within the human brain that is reminiscent of those reported in the single units of rodents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data acquired from the healthy participants are available at Ludwig-Maximilians-Universität via https://data.ub.uni-muenchen.de/439/ (ref. 87). Due to privacy laws, data acquired from the patients are not openly available, though (subject to privacy laws) can be provided by contacting the corresponding author. Source data are provided with this paper.
Code availability
Openly available at GitHub via https://github.com/benjaminGriffiths/human-hd (ref. 88).
References
Buzsáki, G. & Moser, E. I. Memory, navigation and theta rhythm in the hippocampal–entorhinal system. Nat. Neurosci. 16, 130–138 (2013).
Baumann, O. & Mattingley, J. B. Extrahippocampal contributions to spatial navigation in humans: a review of the neuroimaging evidence. Hippocampus https://doi.org/10.1002/hipo.23313 (2021).
Bellmund, J. L. S., Gärdenfors, P., Moser, E. I. & Doeller, C. F. Navigating cognition: spatial codes for human thinking. Science 362, eaat6766 (2018).
Epstein, R. A., Patai, E. Z., Julian, J. B. & Spiers, H. J. The cognitive map in humans: spatial navigation and beyond. Nat. Neurosci. 20, 1504–1513 (2017).
Geva-Sagiv, M., Las, L., Yovel, Y. & Ulanovsky, N. Spatial cognition in bats and rats: from sensory acquisition to multiscale maps and navigation. Nat. Rev. Neurosci. 16, 94–108 (2015).
Hulse, B. K. & Jayaraman, V. Mechanisms underlying the neural computation of head direction. Annu. Rev. Neurosci. https://doi.org/10.1146/annurev-neuro-072116-031516 (2019).
Long, X. & Zhang, S. J. A novel somatosensory spatial navigation system outside the hippocampal formation. Cell Res. https://doi.org/10.1038/s41422-020-00448-8 (2021).
Muller, R. U., Ranck, J. B. & Taube, J. S. Head direction cells: properties and functional significance. Curr. Opin. Neurobiol. 6, 196–206 (1996).
Ranck, J. B. Head direction cells in the deep layer of dorsal presubiculum in freely moving rats. Soc. Neuroscience Abstr. 10, 599 (1984).
Sharp, P. E., Blair, H. T. & Cho, J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci. 24, 289–294 (2001).
Taube, J. S. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181–207 (2007).
Taube, J. S. & Bassett, J. P. Persistent neural activity in head direction cells. Cereb. Cortex 13, 1162–1172 (2003).
Taube, J. S., Muller, R. & Ranck, J. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435 (1990).
Taube, J. S. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J. Neurosci. 15, 70–86 (1995).
Taube, J. S. & Muller, R. U. Comparisons of head direction cell activity in the postsubiculum and anterior thalamus of freely moving rats. Hippocampus 8, 87–108 (1998).
Goodridge, J. P., Dudchenko, P. A., Worboys, K. A., Golob, E. J. & Taube, J. S. Cue control and head direction cells. Behav. Neurosci. 112, 749–761 (1998).
Blair, H. T. & Sharp, P. Anticipatory head direction signals in anterior thalamus: evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J. Neurosci. 15, 6260–6270 (1995).
Zirkelbach, J., Stemmler, M. & Herz, A. V. M. Anticipatory neural activity improves the decoding accuracy for dynamic head-direction signals. J. Neurosci. 39, 2847–2859 (2019).
Butler, W. N., Smith, K. S., van der Meer, M. A. A. & Taube, J. S. The head-direction signal plays a functional role as a neural compass during navigation. Curr. Biol. 27, 1259–1267 (2017).
Calton, J. L. et al. Hippocampal place cell instability after lesions of the head direction cell network. J. Neurosci. 23, 9719–9731 (2003).
Winter, S. S., Clark, B. J. & Taube, J. S. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science 347, 870–874 (2015).
Harland, B. et al. Lesions of the head direction cell system increase hippocampal place field repetition. Curr. Biol. 27, 2706–2712.e2 (2017).
Ajabi, Z., Keinath, A. T., Wei, X.-X. & Brandon, M. P. Population dynamics of head-direction neurons during drift and reorientation. Nature 615, 892–899 (2023).
Chaudhuri, R., Gerçek, B., Pandey, B., Peyrache, A. & Fiete, I. The intrinsic attractor manifold and population dynamics of a canonical cognitive circuit across waking and sleep. Nat. Neurosci. 22, 1512–1520 (2019).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Kunz, L. et al. Mesoscopic neural representations in spatial navigation. Trends Cogn. Sci. 23, 615–630 (2019).
Nau, M., Navarro Schröder, T., Frey, M. & Doeller, C. F. Behavior-dependent directional tuning in the human visual-navigation network. Nat. Commun. 11, 1–13 (2020).
Stangl, M., Maoz, S. L. & Suthana, N. Mobile cognition: imaging the human brain in the ‘real world’. Nat. Rev. Neurosci. 24, 347–362 (2023).
Steel, A., Robertson, C. E. & Taube, J. S. Current promises and limitations of combined virtual reality and functional magnetic resonance imaging research in humans: a commentary on Huffman and Ekstrom (2019). J. Cogn. Neurosci. 33, 159–166 (2021).
Taube, J. S., Valerio, S. & Yoder, R. M. Is navigation in virtual reality with fMRI really navigation? J. Cogn. Neurosci. 25, 1008–1019 (2013).
Do, T.-T. N., Lin, C.-T. & Gramann, K. Human brain dynamics in active spatial navigation. Sci Rep. 11, 13036 (2021).
Gramann, K., Hohlefeld, F. U., Gehrke, L. & Klug, M. Human cortical dynamics during full-body heading changes. Sci Rep. 11, 18186 (2021).
Griffiths, B. J., Mazaheri, A., Debener, S. & Hanslmayr, S. Brain oscillations track the formation of episodic memories in the real world. NeuroImage 143, 256–266 (2016).
Maoz, S. L. L. et al. Dynamic neural representations of memory and space during human ambulatory navigation. Nat. Commun. 14, 6643 (2023).
Piñeyro Salvidegoitia, M. et al. Out and about: subsequent memory effect captured in a natural outdoor environment with smartphone EEG. Psychophysiology https://doi.org/10.1111/psyp.13331 (2019).
Schreiner, T. et al. Memory reactivation of real-world spatial orientation revealed by human electrophysiology. Preprint at bioRxiv https://doi.org/10.1101/2023.01.27.525854 (2023).
Giocomo, L. M. et al. Topography of head direction cells in medial entorhinal cortex. Curr. Biol. 24, 252–262 (2014).
Wirth, S., Baraduc, P., Planté, A., Pinède, S. & Duhamel, J.-R. Gaze-informed, task-situated representation of space in primate hippocampus during virtual navigation. PLoS Biol. 15, e2001045 (2017).
Martinez-Trujillo, J., Piza, D., Corrigan, B., Gulli, R., Do Carmo, S., Cuello, A. C. & Muller, L. Primacy of vision shapes behavioral strategies and neural substrates of spatial navigation in the hippocampus of the common marmoset. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3231892/v1 (2023).
Kriegeskorte, N. Representational similarity analysis—connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 1–28 (2008).
Cullen, K. E. & Taube, J. S. Our sense of direction: progress, controversies and challenges. Nat. Neurosci. 20, 1465–1473 (2017).
Bernardi, S. et al. The geometry of abstraction in the hippocampus and prefrontal cortex. Cell 183, 954–967.e21 (2020).
Dudchenko, P. A., Wood, E. R. & Smith, A. A new perspective on the head direction cell system and spatial behavior. Neurosci. Biobehav. Rev. 105, 24–33 (2019).
Patai, E. Z. & Spiers, H. J. The versatile wayfinder: prefrontal contributions to spatial navigation. Trends Cogn. Sci. 25, 520–533 (2021).
Jacob, P.-Y. et al. An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nat. Neurosci. 20, 173–175 (2017).
Lomi, E., Jeffery, K. J. & Mitchell, A. S. Convergence of location, direction, and theta in the rat anteroventral thalamic nucleus. Iscience 26, 106993 (2023).
Calton, J. L., Turner, C. S., Cyrenne, D.-L. M., Lee, B. R. & Taube, J. S. Landmark control and updating of self-movement cues are largely maintained in head direction cells after lesions of the posterior parietal cortex. Behav. Neurosci. 122, 827–840 (2008).
Medendorp, W. P., Beurze, S. M., Van Pelt, S. & Van Der Werf, J. Behavioral and cortical mechanisms for spatial coding and action planning. Cortex 44, 587–597 (2008).
Blair, H. T., Cho, J. & Sharp, P. E. Role of the lateral mammillary nucleus in the rat head direction circuit: a combined single unit recording and lesion study. Neuron 21, 1387–1397 (1998).
Stackman, R. W. & Taube, J. S. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J. Neurosci. 18, 9020–9037 (1998).
Bassett, J. P. & Taube, J. S. Neural correlates for angular head velocity in the rat dorsal tegmental nucleus. J. Neurosci. 21, 5740–5751 (2001).
Shinder, M. E. & Taube, J. S. Self-motion improves head direction cell tuning. J. Neurophysiol. 111, 2479–2492 (2014).
Shine, J. P., Valdés-Herrera, J. P., Hegarty, M. & Wolbers, T. The human retrosplenial cortex and thalamus code head direction in a global reference frame. J. Neurosci. 36, 6371–6381 (2016).
Shinder, M. E. & Taube, J. S. Active and passive movement are encoded equally by head direction cells in the anterodorsal thalamus. J. Neurophysiol. 106, 788–800 (2011).
Laurens, J., Kim, B., Dickman, J. D. & Angelaki, D. E. Gravity orientation tuning in macaque anterior thalamus. Nat. Neurosci. 19, 1566–1568 (2016).
Robertson, R. G., Rolls, E. T., Georges-François, P. & Panzeri, S. Head direction cells in the primate pre-subiculum. Hippocampus 9, 206–219 (1999).
Georges-Francois, P. Spatial view cells in the primate hippocampus: allocentric view not head direction or eye position or place. Cereb. Cortex 9, 197–212 (1999).
Julian, J. B., Keinath, A. T., Frazzetta, G. & Epstein, R. A. Human entorhinal cortex represents visual space using a boundary-anchored grid. Nat. Neurosci. 21, 191–194 (2018).
Killian, N. J., Jutras, M. J. & Buffalo, E. A. A map of visual space in the primate entorhinal cortex. Nature 491, 761–764 (2012).
Meister, M. L. R. & Buffalo, E. A. Neurons in primate entorhinal cortex represent gaze position in multiple spatial reference frames. J. Neurosci. 38, 2430–2441 (2018).
Nau, M., Navarro Schröder, T., Bellmund, J. L. S. & Doeller, C. F. Hexadirectional coding of visual space in human entorhinal cortex. Nat. Neurosci. 21, 188–190 (2018).
Rolls, E. T. Spatial view cells and the representation of place in the primate hippocampus. Hippocampus 9, 467–480 (1999).
Rolls, E. T. & O’Mara, S. M. View-responsive neurons in the primate hippocampal complex. Hippocampus 5, 409–424 (1995).
Staudigl, T. et al. Hexadirectional modulation of high-frequency electrophysiological activity in the human anterior medial temporal lobe maps visual space. Curr. Biol. 28, 3325–3329.e4 (2018).
Killian, N. J., Potter, S. M. & Buffalo, E. A. Saccade direction encoding in the primate entorhinal cortex during visual exploration. Proc. Natl Acad. Sci. USA 112, 15743–15748 (2015).
Cowie, R. J. & Robinson, D. L. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J. Neurophysiol. 72, 2648–2664 (1994).
Elsley, J. K., Nagy, B., Cushing, S. L. & Corneil, B. D. Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. J. Neurophysiol. 98, 1333–1354 (2007).
Goonetilleke, S. C., Gribble, P. L., Mirsattari, S. M., Doherty, T. J. & Corneil, B. D. Neck muscle responses evoked by transcranial magnetic stimulation of the human frontal eye fields: TMS of the FEF evokes neck muscle activity. Eur. J. Neurosci. 33, 2155–2167 (2011).
Gu, C. & Corneil, B. D. Transcranial magnetic stimulation of the prefrontal cortex in awake nonhuman primates evokes a polysynaptic neck muscle response that reflects oculomotor activity at the time of stimulation. J. Neurosci. 34, 14803–14815 (2014).
Sparks, D. L., Freedman, E. G., Chen, L. L. & Gandhi, N. J. Cortical and subcortical contributions to coordinated eye and head movements. Vision Res. 41, 3295–3305 (2001).
Baumann, O. & Mattingley, J. B. Medial parietal cortex encodes perceived heading direction in Humans. J. Neurosci. 30, 12897–12901 (2010).
Jacobs, J., Kahana, M. J., Ekstrom, A. D., Mollison, M. V. & Fried, I. A sense of direction in human entorhinal cortex. Proc. Natl Acad. Sci USA 107, 6487–6492 (2010).
Kim, M. & Maguire, E. A. Encoding of 3D head direction information in the human brain. Hippocampus 29, 619–629 (2019).
Kunz, L. et al. A neural code for egocentric spatial maps in the human medial temporal lobe. Neuron 109, 2781–2796.e10 (2021).
Vass, L. K. & Epstein, R. A. Abstract representations of location and facing direction in the human brain. J. Neurosci. 33, 6133–6142 (2013).
Stackman, R. W. & Taube, J. S. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J. Neurosci. 17, 4349–4358 (1997).
Yoder, R. M. et al. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. J. Neurophysiol. 105, 2989–3001 (2011).
Gramann, K., Ferris, D. P., Gwin, J. & Makeig, S. Imaging natural cognition in action. Int. J. Psychophysiol. 91, 22–29 (2014).
Lu, Z., Julian, J. B. & Epstein, R. A. Coding of head direction in the human visual system during dynamic navigation. J. Vis. 22, 4277–4277 (2022).
Stangl, M. et al. Boundary-anchored neural mechanisms of location-encoding for self and others. Nature 589, 420–425 (2021).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 1–9 (2011).
Horn, A. & Kühn, A. A. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. NeuroImage 107, 127–135 (2015).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Ashburner, J. & Friston, K. J. Unified segmentation. NeuroImage 26, 839–851 (2005).
Staudigl, T. Data for: Griffiths et al.: Electrophysiological signatures of veridical head direction in humans (Nature Human Behaviour, 2024). (Open Data LMU, 2024). https://doi.org/10.5282/ubm/data.439
Acknowledgements
This work was supported by the European Research Council (https://erc.europa.eu/, starting grant 802681 awarded to T. Schreiner) and the Leverhulme Trust (https://www.leverhulme.ac.uk/, early career fellowship ECF-2021-628 awarded to B.J.G.). We thank all participants and in particular all patients who volunteered to participate in this study. We thank the staff and physicians at the Epilepsy Center, Department of Neurology, Ludwig-Maximilians-Universität, Munich, for assistance. We thank A. Chowdhury for valuable input. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
B.J.G.: conceptualization, methodology, software, validation and formal analysis; investigation; data curation; writing—original draft; writing—review and editing; and visualization. T. Schreiner: conceptualization, methodology, investigation, data curation and writing—review and editing. J.K.S.: investigation, data curation and writing—review and editing. C.V.: resources and writing—review and editing. E.K.: resources and writing—review and editing. S.Q.: resources and writing—review and editing. J.R.: resources and writing—review and editing. S.N.: resources and writing—review and editing. T. Staudigl: conceptualization, methodology and investigation; writing—original draft; and writing—review and editing, supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Liang Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–7 and Figs. 1–22.
Source data
Source Data Figs. 1–6
Statistical source data. Supplementary Data 1 contains all source data for plots in the main text.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Griffiths, B.J., Schreiner, T., Schaefer, J.K. et al. Electrophysiological signatures of veridical head direction in humans. Nat Hum Behav (2024). https://doi.org/10.1038/s41562-024-01872-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41562-024-01872-1