Abstract
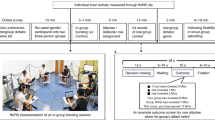
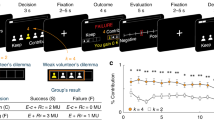
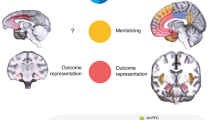
Leaders can launch hostile attacks on out-groups and organize in-group defence. Whether groups settle the conflict in their favour depends, however, on whether followers align with leader’s initiatives. Yet how leader and followers coordinate during intergroup conflict remains unknown. Participants in small groups elected a leader and made costly contributions to intergroup conflict while dorsolateral prefrontal cortex (DLPFC) activity was simultaneously measured. Leaders were more sacrificial and their contribution influenced group survival to a greater extent during in-group defence than during out-group attacks. Leaders also had increased DLPFC activity when defending in-group, which predicted their comparatively strong contribution to conflict; followers reciprocated their leader’s initiatives the more their DLPFC activity synchronized with that of their leader. When launching attacks, however, leaders and followers aligned poorly at behavioural and neural levels, which explained why out-group attacks often failed. Our results provide a neurobehavioural account of leader–follower coordination during intergroup conflict and reveal leader–follower behavioural/neural alignment as pivotal for groups settling conflicts in their favour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All behavioural data and materials have been made publicly available via the Open Science Framework and can be accessed at https://osf.io/7grfu/. The neural data supporting the main findings of this study are available from the corresponding author upon request.
Code availability
The custom routines for the main data analysis written in MATLAB are available in the Open Science Framework at https://osf.io/7grfu/.
References
Ma, Y. & Tan, H. Representation of intergroup conflict in the human brain. Neuron 111, 1692–1696 (2023).
Glowacki, L. & McDermott, R. Key individuals catalyse intergroup violence. Phil. Trans. R. Soc. Lond. B 377, 20210141 (2022).
De Dreu, C. K. W., Fariña, A., Gross, J. & Romano, A. Prosociality as a foundation for intergroup conflict. Curr. Opin. Psychol. 44, 112–116 (2022).
Smith, J. E. et al. Sex bias in intergroup conflict and collective movements among social mammals: male warriors and female guides. Phil. Trans. R. Soc. Lond. B 377, 20210142 (2022).
von Rueden, C., Gurven, M., Kaplan, H. & Stieglitz, J. Leadership in an egalitarian society. Hum. Nat. 25, 538–566 (2014).
Benard, S. Cohesion from conflict: does intergroup conflict motivate intragroup norm enforcement and support for centralized leadership? Soc. Psychol. Q. 75, 107–130 (2012).
Gavrilets, S. & Fortunato, L. A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 3526 (2014).
De Dreu, C. K. W. et al. In-group defense, out-group aggression, and coordination failures in intergroup conflict. Proc. Natl Acad. Sci. USA 113, 10524–10529 (2016).
Zhang, H., Gross, J., De Dreu, C. K. & Ma, Y. Oxytocin promotes coordinated out-group attack during intergroup conflict in humans. eLife 8, e40698 (2019).
Mathew, S. & Boyd, R. The cost of cowardice: punitive sentiments towards free riders in Turkana raids. Evol. Hum. Behav. 35, 58–64 (2014).
De Dreu, C. K. W. & Triki, Z. Intergroup conflict: origins, dynamics and consequences across taxa. Phil. Trans. R. Soc. Lond. B 377, 20210134 (2022).
King, M. L. Jr. Where Do We Go From Here: Chaos or Community? Vol. 2 (Beacon Press, 2010).
Blom, M. & Alvesson, M. Leadership on demand: followers as initiators and inhibitors of managerial leadership. Scand. J. Manage. 30, 344–357 (2014).
Glowacki, L. et al. Formation of raiding parties for intergroup violence is mediated by social network structure. Proc. Natl Acad. Sci. USA 113, 12114–12119 (2016).
Stein, R. M. War and revenge: explaining conflict initiation by democracies. Am. Polit. Sci. Rev. 109, 556–573 (2015).
Baum, M. A. & Potter, P. B. The relationships between mass media, public opinion, and foreign policy: toward a theoretical synthesis. Annu. Rev. Polit. Sci. 11, 39–65 (2008).
Dogan, G., Glowacki, L. & Rusch, H. Spoils division rules shape aggression between natural groups. Nat. Hum. Behav. 2, 322–326 (2018).
Johnstone, R. A., Cant, M. A., Cram, D. & Thompson, F. J. Exploitative leaders incite intergroup warfare in a social mammal. Proc. Natl Acad. Sci. USA 117, 29759–29766 (2020).
Sankey, D. W. E. et al. Leaders of war: modelling the evolution of conflict among heterogeneous groups. Phil. Trans. R. Soc. Lond. B 377, 20210140 (2022).
De Dreu, C. K. W., Gross, J. & Reddmann, L. Environmental stress increases out-group aggression and intergroup conflict in humans. Phil. Trans. R. Soc. Lond. B 377, 20210147 (2022).
Weisel, O. & Zultan, R. I. Perceived level of threat and cooperation. Front. Psychol. 12, 704338 (2021).
De Dreu, C. K. W. & Gross, J. Revisiting the form and function of conflict: neurobiological, psychological, and cultural mechanisms for attack and defense within and between groups. Behav. Brain Sci. 42, e116 (2019).
Stein, A. A. Conflict and cohesion: a review of the literature. J. Conflict Resolut. 20, 143–172 (1976).
Lojowska, M., Gross, J. & De Dreu, C. K. W. Anticipatory threat mitigates the breakdown of group cooperation. Psychol. Sci. 34, 87–98 (2022).
Kritzinger, S. et al. ‘Rally round the flag’: the COVID-19 crisis and trust in the national government. West Eur. Polit. 44, 1205–1231 (2021).
Sumner, W. G. Folkways: The Sociological Importance of Usages, Manners, Customs, Mores and Morals (Ginn and Co., 1907).
Williams, R. M. Jr. The Reduction of Intergroup Tensions: A Survey of Research on Problems of Ethnic, Racial, and Religious Group Relations (Social Science Research Council, 1947).
Gneezy, A. & Fessler, D. M. Conflict, sticks and carrots: war increases prosocial punishments and rewards. Phil. Trans. R. Soc. Lond. B 279, 219–223 (2012).
Davis, D. W. & Silver, B. D. Civil liberties vs. security: public opinion in the context of the terrorist attacks on America. Am. J. Polit. Sci. 48, 28–46 (2004).
Smith, J. E. et al. Leadership in mammalian societies: emergence, distribution, power, and payoff. Trends Ecol. Evol. 31, 54–66 (2016).
Yam, K. C. et al. The rise of COVID-19 cases is associated with support for world leaders. Proc. Natl Acad. Sci. USA 117, 25429–25433 (2020).
Jiang, J. et al. Leader emergence through interpersonal neural synchronization. Proc. Natl Acad. Sci. USA 112, 4274–4279 (2015).
Yang, J., Zhang, H., Ni, J., De Dreu, C. K. W. & Ma, Y. Within-group synchronization in the prefrontal cortex associates with intergroup conflict. Nat. Neurosci. 23, 754–760 (2020).
Gamliel, H. N. et al. Inter-group conflict affects inter-brain synchrony during synchronized movements. Neuroimage 245, 118661 (2021).
Suzuki, S., Jensen, E. L., Bossaerts, P. & O’Doherty, J. P. Behavioral contagion during learning about another agent’s risk-preferences acts on the neural representation of decision-risk. Proc. Natl Acad. Sci. USA 113, 3755–3760 (2016).
Huber, R. E., Klucharev, V. & Rieskamp, J. Neural correlates of informational cascades: brain mechanisms of social influence on belief updating. Soc. Cogn. Affect. Neurosci. 10, 589–597 (2015).
Spitzer, M., Fischbacher, U., Herrnberger, B., Grön, G. & Fehr, E. The neural signature of social norm compliance. Neuron 56, 185–196 (2007).
Stallen, M. et al. Neurobiological mechanisms of responding to injustice. J. Neurosci. 38, 2944–2954 (2018).
Zinchenko, O. & Arsalidou, M. Brain responses to social norms: meta-analyses of fMRI studies. Hum. Brain Mapp. 39, 955–970 (2018).
Fairhurst, M. T., Janata, P. & Keller, P. E. Being and feeling in sync with an adaptive virtual partner: brain mechanisms underlying dynamic cooperativity. Cereb. Cortex 23, 2592–2600 (2013).
Ligneul, R., Girard, R. & Dreher, J. C. Social brains and divides: the interplay between social dominance orientation and the neural sensitivity to hierarchical ranks. Sci. Rep. 7, 45920 (2017).
Qu, C., Ligneul, R., Van der Henst, J. B. & Dreher, J. C. An integrative interdisciplinary perspective on social dominance hierarchies. Trends Cogn. Sci. 21, 893–908 (2017).
Saxe, R. & Kanwisher, N. People thinking about thinking people: the role of the temporo-parietal junction in ‘theory of mind’. Neuroimage 19, 1835–1842 (2003).
Schurz, M., Radua, J., Aichhorn, M., Richlan, F. & Perner, J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 42, 9–34 (2014).
Carter, M. C., Bowling, D. L., Reeck, C. & Huettel, S. A. A distinct role of the temporo-parietal junction in predicting socially guided decisions. Science 337, 109–111 (2012).
Shamay-Tsoory, S. G., Saporta, N., Marton-Alper, I. Z. & Gvirts, H. Z. Herding brains: a core neural mechanism for social alignment. Trends Cogn. Sci. 23, 174–186 (2019).
Dai, B. et al. Neural mechanisms for selectively tuning in to the target speaker in a naturalistic noisy situation. Nat. Commun. 9, 2405 (2018).
Brooks, J., Onishi, E., Clark, I. R., Bohn, M. & Yamamoto, S. Uniting against a common enemy: perceived outgroup threat elicits ingroup cohesion in chimpanzees. PLoS ONE 16, e0246869 (2021).
Braga Goncalves, I. & Radford, A. N. Experimental evidence that intruder and group member attributes affect outgroup defence and associated within-group interactions in a social fish. Phil. Trans. R. Soc. Lond. B 286, 20191261 (2019).
Mu, Y., Han, S. & Gelfand, M. J. The role of gamma interbrain synchrony in social coordination when humans face territorial threats. Soc. Cogn. Affect. Neurosci. 12, 1614–1623 (2017).
Hosokawa, T., Kennerley, S. W., Sloan, J. & Wallis, J. D. Single-neuron mechanisms underlying cost-benefit analysis in frontal cortex. J. Neurosci. 33, 17385–17397 (2013).
Hu, Y. et al. Perturbation of right dorsolateral prefrontal cortex (rDLPFC) makes power-holders less resistant to tempting bribes. Psychol. Sci. 33, 412–423 (2021).
Shackman, A. J., McMenamin, B. W., Maxwell, J. S., Greischar, L. L. & Davidson, R. J. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychol. Sci. 20, 1500–1506 (2009).
Steinbeis, N., Bernhardt, B. C. & Singer, T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron 73, 1040–1051 (2012).
Balderston, N. L., Hsiung, A., Ernst, M. & Grillon, C. Effect of threat on right dlPFC activity during behavioral pattern separation. J. Neurosci. 37, 9160–9171 (2017).
Mobbs, D. et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317, 1079–1083 (2007).
Smith, E. H. et al. Widespread temporal coding of cognitive control in the human prefrontal cortex. Nat. Neurosci. 22, 1883–1891 (2019).
van Dijk, E., De Dreu, C. K. W. & Gross, J. Power in economic games. Curr. Opin. Psychol. 33, 100–104 (2020).
Osaka, N. et al. How two brains make one synchronized mind in the inferior frontal cortex: fNIRS-based hyperscanning during cooperative singing. Front. Psychol. 6, 1811 (2015).
Tang, H. et al. Interpersonal brain synchronization in the right temporo-parietal junction during face-to-face economic exchange. Soc. Cogn. Affect. Neurosci. 11, 23–32 (2016).
Obrig, H. & Villringer, A. Beyond the visible—imaging the human brain with light. J. Cerebr. Blood Flow Metab. 23, 1–18 (2003).
Hoshi, Y., Kohri, S. & Kobayashi, N. in Complex Medical Engineering (eds Wu, J. L. et al.) 469–479 (Springer, 2007).
Hoshi, Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology 40, 511–520 (2003).
Huppert, T. J., Hoge, R. D., Diamond, S. G., Franceschini, M. A. & Boas, D. A. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage 29, 368–382 (2006).
Cui, X., Bray, S., Bryant, D. M., Glover, G. H. & Reiss, A. L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 54, 2808–2821 (2011).
Strangman, G., Culver, J. P., Thompson, J. H. & Boas, D. A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17, 719–731 (2002).
Van Vugt, M. & Smith, J. E. A dual model of leadership and hierarchy: evolutionary synthesis. Trends Cogn. Sci. 23, 952–967 (2019).
Ye, J. C., Tak, S., Jang, K. E., Jung, J. & Jang, J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage 44, 428–447 (2009).
Xue, H., Lu, K. & Hao, N. Cooperation makes two less-creative individuals turn into a highly-creative pair. Neuroimage 172, 527–537 (2018).
Holmes, A. P., Blair, R. C., Watson, J. D. G. & Ford, I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cerebr. Blood Flow Metab. 16, 7–22 (1996).
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25 (2002).
Acknowledgements
We thank C. Yang and X. Zou for assistance in data collection. This work was supported by the National Natural Science Foundation of China (Project 32125019 to Y.M.); the STI 2030—Major Projects 2022ZD0211000 to Y.M.; the Major Project of National Social Science Foundation (19ZDA363 to Y.M.); the Fundamental Research Funds for the Central Universities (to Y.M.); the start-up funding from the State Key Laboratory of Cognitive Neuroscience and Learning, IDG/McGovern Institute for Brain Research, Beijing Normal University (to Y.M.) and the Spinoza Award from the Netherlands Science Foundation (NWO SPI-57-242 to C.K.W.D.D.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.M. and C.K.W.D.D. conceived the project and designed the experiment; H.Z., J.Y. and J.N. implemented the experiment and collected data; H.Z. and J.Y. analysed the data under the supervision of Y.M.; Y.M. and C.K.W.D.D. interpreted the results; Y.M. wrote the original and final version of the manuscript; J.Y. and C.K.W.D.D. provided critical revisions. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Yinying Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Verify frequency band with increased neural synchronization to intergroup contest than resting-state.
To validate that the timeline-based frequency band of interest, we averaged coherence values across all 14 channels and the averaged coherence value during the resting-state was subtracted from that of inter-group contest task. We performed one sample t-tests on the coherence value difference for each of the 121 frequency bands (frequency range: 0.004 to 4.547 Hz). Frequency band from 0.0282 Hz to 0.0335 Hz (corresponding to the period between 29.82s and 35.47s) showed significantly increased coherence value of inter-group contest (vs. resting state, p < 0.05, corrected by False discovery rate (FDR) for 121 frequency bands), which was overlapping with the timeline-based frequency band of interest (that is, frequency band from 0.0263 HZ to 0.0357 HZ). Moreover, the coherence value within the timeline-identified frequency band of interest also showed significant increase during inter-group contest than the resting-state (Mean ± SE = 0.011 ± 0.004, t79 = 2.963, p = 0.004, Cohen’s d = 0.331).
Extended Data Fig. 2 Neural synchronization between the group leader and a randomly selected follower.
There were significant leader-by-role interaction when considering neural synchronization of the leader and a randomly selected follower, rather than the across pair averages (a: channel 11, F1,78 = 9.230, p = 0.003, η2p = 0.106; b: channel 6, F1,78 = 9.219, p = 0.003, η2p = 0.106). Data are shown as the mean ± s.e. with overlaid dot plots. n = 80 three-versus-three-person intergroup contest sessions. **p < 0.01.
Extended Data Fig. 3 Validation of neural synchronization.
(a) We generated pseudo groups by randomly grouping a real leader and two real followers from different original groups to a pseudo group. We then re-calculated neural synchronization of pseudo groups using the same method as we did for the real pairs. We repeated the generation of pseudo groups and recalculation of neural synchronization for 1000 times. We verified stronger neural synchronization in real interacting leader-follower pairs of defender groups than pseudo groups (b, p = 0.003), but not for follower-follower pairs of defender groups (c, p = 0.981), nor leader-follower (d, p = 0.711), follower-follower (e, p = 0.590) pairs of attacker groups.
Extended Data Fig. 4 Validation of neural synchronization.
(a) We kept each original group and generated within-group pseudo pairs for each group by randomly assigning one of the two followers as a pseudo leader. We then re-calculated neural synchronization of pseudo pairs using the same method as we did for the real pairs. We repeated the generation of pseudo pairs and recalculation of neural synchronization for 1000 times. We verified stronger neural synchronization in real interacting leader-follower pairs of defender groups than pseudo groups (b, p = 0.001), but not for follower-follower pairs of defender groups (c, p = 0.999), nor leader-follower (d, p = 0.450), follower-follower (e, p = 0.420) pairs of attacker groups.
Supplementary information
Supplementary Information
Supplementary Tables 1–8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Yang, J., Ni, J. et al. Leader–follower behavioural coordination and neural synchronization during intergroup conflict. Nat Hum Behav 7, 2169–2181 (2023). https://doi.org/10.1038/s41562-023-01663-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-023-01663-0
This article is cited by
-
Arbeit im Team und psychische Gesundheit
Der Nervenarzt (2023)