Abstract
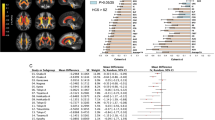
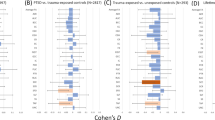
Psychiatric disorders share neurobiology and frequently co-occur. This neurobiological and clinical overlap highlights opportunities for transdiagnostic treatments. In this study, we used coordinate and lesion network mapping to test for a shared brain network across psychiatric disorders. In our meta-analysis of 193 studies, atrophy coordinates across six psychiatric disorders mapped to a common brain network defined by positive connectivity to anterior cingulate and insula, and by negative connectivity to posterior parietal and lateral occipital cortex. This network was robust to leave-one-diagnosis-out cross-validation and specific to atrophy coordinates from psychiatric versus neurodegenerative disorders (72 studies). In 194 patients with penetrating head trauma, lesion damage to this network correlated with the number of post-lesion psychiatric diagnoses. Neurosurgical ablation targets for psychiatric illness (four targets) also aligned with the network. This convergent brain network for psychiatric illness may partially explain high rates of psychiatric comorbidity and could highlight neuromodulation targets for patients with more than one psychiatric disorder.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
This paper used de-identified data from multiple datasets collected by different investigators at different institutions. Datasets 1 (https://doi.org/10.1001/jamapsychiatry.2014.2206), 2 (https://doi.org/10.1093/brain/awy292) and 4 (https://doi.org/10.1038/npp.2010.132) are publicly available peer-reviewed publications. Inquiries regarding the Vietnam Head Injury Study (Dataset 3) can be directed to J.G. (jgrafman@northwestern.edu). The one-sample t-test transdiagnostic network is available at https://github.com/nimlab/NHB_Taylor2023.
Code availability
GingerALE is publicly available. The custom MATLAB and Python code used in this study is available at https://github.com/nimlab/NHB_Taylor2023.
References
Barch, D. M. What does it mean to be transdiagnostic and how would we know? Am. J. Psychiatry 177, 370–372 (2020).
Newman, D. L., Moffitt, T. E., Caspi, A. & Silva, P. A. Comorbid mental disorders: implications for treatment and sample selection. J. Abnorm. Psychol. 107, 305–311 (1998).
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R. & Walters, E. E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627 (2005).
Goldstein-Piekarski, A. N., Williams, L. M. & Humphreys, K. A trans-diagnostic review of anxiety disorder comorbidity and the impact of multiple exclusion criteria on studying clinical outcomes in anxiety disorders. Transl. Psychiatry 6, e847 (2016).
Caspi, A. & Moffitt, T. E. All for one and one for all: mental disorders in one dimension. Am. J. Psychiatry 175, 831–844 (2018).
Grisanzio, K. A. et al. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry 75, 201–209 (2018).
Caspi, A. et al. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin birth cohort study. JAMA Netw. Open 3, e203221 (2020).
Hyman, S. E. The diagnosis of mental disorders: the problem of reification. Annu. Rev. Clin. Psychol. 6, 155–179 (2010).
Kessler, R. C. et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 8–19 (1994).
McGrath, J. J. et al. Comorbidity within mental disorders: a comprehensive analysis based on 145 990 survey respondents from 27 countries. Epidemiol. Psychiatr. Sci. 29, e153 (2020).
Plana-Ripoll, O. et al. Nature and prevalence of combinations of mental disorders and their association with excess mortality in a population-based cohort study. World Psychiatry 19, 339–349 (2020).
Plana-Ripoll, O. et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet 394, 1827–1835 (2019).
Fridell, M. et al. Prediction of psychiatric comorbidity on premature death in a cohort of patients with substance use disorders: a 42-year follow-up. BMC Psychiatry 19, 150 (2019).
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40 (2006).
Saravay, S. M. & Lavin, M. Psychiatric comorbidity and length of stay in the general hospital: a critical review of outcome studies. Psychosomatics 35, 233–252 (1994).
Mojtabai, R. & Olfson, M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch. Gen. Psychiatry 67, 26–36 (2010).
Sareen, J. et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch. Gen. Psychiatry 62, 1249–1257 (2005).
Stein, D. J. Comorbidity in generalized anxiety disorder: impact and implications. J. Clin. Psychiatry 62, 29–34 (2001).
Wittchen, H. U., Zhao, S., Kessler, R. C. & Eaton, W. W. DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 355–364 (1994).
Cacciola, J. S., Alterman, A. I., Rutherford, M. J., McKay, J. R. & Mulvaney, F. D. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 61, 271–280 (2001).
Souery, D. et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J. Clin. Psychiatry 68, 1062–1070 (2007).
Maj, M. “Psychiatric comorbidity”: an artefact of current diagnostic systems? Br. J. Psychiatry 186, 182–184 (2005).
van Loo, H. M., Romeijn, J. W., de Jonge, P. & Schoevers, R. A. Psychiatric comorbidity and causal disease models. Prev. Med. 57, 748–752 (2013).
Caspi, A. et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2, 119–137 (2014).
Moore, T. M. et al. Development of a computerized adaptive screening tool for overall psychopathology (“p”). J. Psychiatr. Res. 116, 26–33 (2019).
Sprooten, E., Franke, B. & Greven, C. U. The P-factor and its genomic and neural equivalents: an integrated perspective. Mol. Psychiatry 27, 38–48 (2022).
Fried, E. I., Greene, A. L. & Eaton, N. R. The p factor is the sum of its parts, for now. World Psychiatry 20, 69–70 (2021).
Huang, J. et al. Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am. J. Psychiatry 167, 1254–1263 (2010).
Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381, 1371–1379 (2013).
Brainstorm, C. et al. Analysis of shared heritability in common disorders of the brain. Science https://doi.org/10.1126/science.aap8757 (2018).
Cross-Disorder Group of the Psychiatric Genomics Consortiumet al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 45, 984–994 (2013).
Sanders, S. J. et al. Whole genome sequencing in psychiatric disorders: the WGSPD consortium. Nat. Neurosci. 20, 1661–1668 (2017).
Hoeffding, L. K. et al. Risk of psychiatric disorders among individuals with the 22q11.2 deletion or duplication: a Danish nationwide, register-based study. JAMA Psychiatry 74, 282–290 (2017).
Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482e1411 (2019).
Hyman, S. E. New evidence for shared risk architecture of mental disorders. JAMA Psychiatry 76, 235–236 (2019).
Plana-Ripoll, O. et al. Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry 76, 259–270 (2019).
Lahey, B. B. et al. Is there a general factor of prevalent psychopathology during adulthood? J. Abnorm. Psychol. 121, 971–977 (2012).
Clark, L. A., Watson, D. & Reynolds, S. Diagnosis and classification of psychopathology: challenges to the current system and future directions. Annu. Rev. Psychol. 46, 121–153 (1995).
Lahey, B. B., Krueger, R. F., Rathouz, P. J., Waldman, I. D. & Zald, D. H. A hierarchical causal taxonomy of psychopathology across the life span. Psychol. Bull. 143, 142–186 (2017).
Krueger, R. F. & Eaton, N. R. Transdiagnostic factors of mental disorders. World Psychiatry 14, 27–29 (2015).
Fitzgerald, P. B. Targeting repetitive transcranial magnetic stimulation in depression: do we really know what we are stimulating and how best to do it? Brain Stimul. 14, 730–736 (2021).
Siddiqi, S. H., Weigand, A., Pascual-Leone, A. & Fox, M. D. Identification of personalized transcranial magnetic stimulation targets based on subgenual cingulate connectivity: an independent replication. Biol. Psychiatry 90, e55–e56 (2021).
Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 (2020).
Cash, R. F. H. et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol. Psychiatry 90, 689–700 (2020).
Stelten, B. M., Noblesse, L. H., Ackermans, L., Temel, Y. & Visser-Vandewalle, V. The neurosurgical treatment of addiction. Neurosurg. Focus 25, E5 (2008).
Schoene-Bake, J. C. et al. Tractographic analysis of historical lesion surgery for depression. Neuropsychopharmacology 35, 2553–2563 (2010).
Patel, S. R., Aronson, J. P., Sheth, S. A. & Eskandar, E. N. Lesion procedures in psychiatric neurosurgery. World Neurosurg. 80, S31.e9–S31.e16 (2013).
Opel, N. et al. Cross-disorder analysis of brain structural abnormalities in six major psychiatric disorders: a secondary analysis of mega- and meta-analytical findings from the ENIGMA consortium. Biol. Psychiatry 88, 678–686 (2020).
Eckstrand, K. L. Shared versus disorder-specific brain morphometric features of major psychiatric disorders in adulthood. Biol. Psychiatry 88, e41–e43 (2020).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015).
McTeague, L. M. et al. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry 174, 676–685 (2017).
Mitelman, S. A. Transdiagnostic neuroimaging in psychiatry: a review. Psychiatry Res. 277, 23–38 (2019).
Zhukovsky, P. et al. Coordinate-based network mapping of brain structure in major depressive disorder in younger and older adults: a systematic review and meta-analysis. Am. J. Psychiatry 178, 1119–1128 (2021).
Darby, R. R., Joutsa, J. & Fox, M. D. Network localization of heterogeneous neuroimaging findings. Brain 142, 70–79 (2019).
Weil, R. S., Hsu, J. K., Darby, R. R., Soussand, L. & Fox, M. D. Neuroimaging in Parkinson’s disease dementia: connecting the dots. Brain Commun. 1, fcz006 (2019).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Taylor, J. J., Siddiqi, S. H. & Fox, M. D. Coordinate network mapping: an emerging approach for morphometric meta-analysis. Am. J. Psychiatry 178, 1080–1081 (2021).
Eickhoff, S. B. et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926 (2009).
Siddiqi, S. H., Kording, K. P., Parvizi, J. & Fox, M. D. Causal mapping of human brain function. Nat. Rev. Neurosci. 23, 361–375 (2022).
Etkin, A. Addressing the causality gap in human psychiatric neuroscience. JAMA Psychiatry 75, 3–4 (2017).
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C. & Yeo, B. T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
Elliott, M. L., Romer, A., Knodt, A. R. & Hariri, A. R. A connectome-wide functional signature of transdiagnostic risk for mental illness. Biol. Psychiatry 84, 452–459 (2018).
Taquet, M. et al. A structural brain network of genetic vulnerability to psychiatric illness. Mol. Psychiatry 26, 2089–2100 (2021).
Sheffield, J. M. et al. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry 74, 605–613 (2017).
Barch, D. M. The neural correlates of transdiagnostic dimensions of psychopathology. Am. J. Psychiatry 174, 613–615 (2017).
Sharma, A. et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am. J. Psychiatry 174, 657–666 (2017).
McTeague, L. M., Goodkind, M. S. & Etkin, A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 83, 37–46 (2016).
Romer, A. L. et al. Pervasively thinner neocortex as a transdiagnostic feature of general psychopathology. Am. J. Psychiatry 178, 174–182 (2021).
McTeague, L. M. et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 177, 411–421 (2020).
Romer, A. L. et al. Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Mol. Psychiatry 26, 3839–3846 (2021).
Romer, A. L. et al. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol. Psychiatry 23, 1084–1090 (2018).
Hamilton, J. P. et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry 169, 693–703 (2012).
Gursel, D. A., Avram, M., Sorg, C., Brandl, F. & Koch, K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 87, 151–160 (2018).
Patel, R., Spreng, R. N., Shin, L. M. & Girard, T. A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 36, 2130–2142 (2012).
Peters, S. K., Dunlop, K. & Downar, J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 10, 104 (2016).
Rushworth, M. F., Paus, T. & Sipila, P. K. Attention systems and the organization of the human parietal cortex. J. Neurosci. 21, 5262–5271 (2001).
Arnsten, A. F. & Rubia, K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 51, 356–367 (2012).
Lee, K. H. et al. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage 29, 578–586 (2006).
Moran, J. & Desimone, R. Selective attention gates visual processing in the extrastriate cortex. Science 229, 782–784 (1985).
Tallon-Baudry, C., Bertrand, O., Henaff, M. A., Isnard, J. & Fischer, C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb. Cortex 15, 654–662 (2005).
Karten, A., Pantazatos, S. P., Khalil, D., Zhang, X. & Hirsch, J. Dynamic coupling between the lateral occipital-cortex, default-mode, and frontoparietal networks during bistable perception. Brain Connect. 3, 286–293 (2013).
Grill-Spector, K., Kourtzi, Z. & Kanwisher, N. The lateral occipital complex and its role in object recognition. Vis. Res. 41, 1409–1422 (2001).
Plewan, T., Weidner, R., Eickhoff, S. B. & Fink, G. R. Ventral and dorsal stream interactions during the perception of the Muller–Lyer illusion: evidence derived from fMRI and dynamic causal modeling. J. Cogn. Neurosci. 24, 2015–2029 (2012).
Vander Wyk, B. C. et al. Cortical integration of audio-visual speech and non-speech stimuli. Brain Cogn. 74, 97–106 (2010).
Naumer, M. J. et al. Visuohaptic convergence in a corticocerebellar network. Eur. J. Neurosci. 31, 1730–1736 (2010).
Moberget, T. et al. Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol. Psychiatry 86, 65–75 (2019).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Arnone, D. et al. State-dependent changes in hippocampal grey matter in depression. Mol. Psychiatry 18, 1265–1272 (2013).
Rashidi-Ranjbar, N. et al. Frontal-executive and corticolimbic structural brain circuitry in older people with remitted depression, mild cognitive impairment, Alzheimer’s dementia, and normal cognition. Neuropsychopharmacology 45, 1567–1578 (2020).
Nuninga, J. O., Mandl, R. C. W. & Sommer, I. E. C. Clinical relevance of brain changes after electroconvulsive therapy: is there really no link at all? Biol. Psychiatry 89, e13–e14 (2021).
Marinescu, I. E., Lawlor, P. N. & Kording, K. P. Quasi-experimental causality in neuroscience and behavioural research. Nat. Hum. Behav. 2, 891–898 (2018).
Etkin, A. A reckoning and research agenda for neuroimaging in psychiatry. Am. J. Psychiatry 176, 507–511 (2019).
Lariviere, S. et al. Network-based atrophy modeling in the common epilepsies: a worldwide ENIGMA study. Sci. Adv. https://doi.org/10.1126/sciadv.abc6457 (2020).
Whelan, C. D. et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 141, 391–408 (2018).
Galovic, M. et al. Resective surgery prevents progressive cortical thinning in temporal lobe epilepsy. Brain 143, 3262–3272 (2020).
Keramatian, K., Su, W., Saraf, G., Chakrabarty, T. & Yatham, L. N. Preservation of gray matter volume in early stage of bipolar disorder: a case for early intervention. Can. J. Psychiatry 66, 139–146 (2021).
Moylan, S., Maes, M., Wray, N. R. & Berk, M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol. Psychiatry 18, 595–606 (2013).
Fineberg, N. A. et al. Early intervention for obsessive compulsive disorder: an expert consensus statement. Eur. Neuropsychopharmacol. 29, 549–565 (2019).
Ganos, C. et al. A neural network for tics: insights from causal brain lesions and deep brain stimulation. Brain https://doi.org/10.1093/brain/awac009 (2022).
Siddiqi, S. H. et al. Convergent causal mapping of human neuropsychiatric symptoms using brain stimulation and brain lesions. Nat. Hum. Behav. (in the press).
Reich, M. M. et al. A brain network for deep brain stimulation induced cognitive decline in Parkinson’s disease. Brain 145, 1410–1421 (2022).
Weigand, A. et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry 84, 28–37 (2018).
Grafman, J., Salazar, A. M., Weingartner, H. & Amin, D. Face memory and discrimination: an analysis of the persistent effects of penetrating brain wounds. Int. J. Neurosci. 29, 125–139 (1986).
Raymont, V., Salazar, A. M., Krueger, F. & Grafman, J. “Studying injured minds”—the Vietnam head injury study and 40 years of brain injury research. Front. Neurol. 2, 15 (2011).
Koenigs, M. et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat. Neurosci. 11, 232–237 (2008).
Padmanabhan, J. L. et al. A human depression circuit derived from focal brain lesions. Biol. Psychiatry 86, 749–758 (2019).
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F. & Fox, P. T. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361 (2012).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M. & Nichols, T. E. Permutation inference for the general linear model. Neuroimage 92, 381–397 (2014).
Ferguson, M. A. et al. A human memory circuit derived from brain lesions causing amnesia. Nat. Commun. 10, 3497 (2019).
Acknowledgements
We acknowledge the authors of Schoene-Bake et al.46, who provided us with simulated lesions from their work. We also thank W. Drew and J. Li for their technical support and P. Flynn for his administrative support. The authors received no specific funding for this work. J.J.T.: Harvard Medical School (Dupont Warren Fellowship Award and Livingston Award), Brain and Behavior Research Foundation Young Investigator Grant (no. 31081), Sidney R. Baer, Jr. Foundation, Baszucki Brain Research Fund, and the NIH (grant nos K23MH129829 and R01MH113929). C.L.: none. D.T.: NIMH T32 fellowship (no. T32MH020004) and Harvard Medical School (Dupont Warren Fellowship Award). M.A.F.: none. F.L.W.V.J.S.: Epilepsy Society (grant no. 846534). J.J.: Brain and Behavior Research Foundation Young Investigator Grant (no. 29441). M.G.: none. J.G.: none. A.E.: none. S.H.S.: NIH (grant nos K23MH121657 and R21MH126271), Brain and Behavior Research Foundation Young Investigator Grant, Neuronetics investigator-initiated grant, Baszucki Brain Research Fund, and Department of Veterans Affairs (grant no. I01CX002293). M.D.F.: the Nancy Lurie Marks Foundation, the Kaye Family Research Endowment, Baszucki Brain Research Fund and the NIH (grant nos R01MH113929, R21MH126271, R56AG069086, R01MH115949 and R01AG060987).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: J.J.T., D.T., S.H.S. and M.D.F. Design of the analytical procedures: J.J.T., F.L.W.V.J.S., M.A.F., S.H.S. and M.D.F. Preprocessing and preparation of the data for the analyses: J.J.T., C.L., D.T., M.A.F., F.L.W.V.J.S., J.J., M.G., J.G., A.E., S.H.S. and M.D.F. Neuroimaging analyses and statistical analyses: J.J.T., C.L., M.A.F., F.L.W.V.J.S., J.J., S.H.S. and M.D.F. Contribution of the data: M.G., J.G., A.E., S.H.S. and M.D.F. Interpretation of the analyses and writing of the manuscript: J.J.T., S.H.S. and M.D.F., with input from all authors.
Corresponding author
Ethics declarations
Competing interests
J.J.T.: none. C.L.: none. D.T.: none. M.A.F.: none. F.L.W.V.J.S.: none. J.J.: none. M.G.: none. J.G.: none. A.E.: salary and equity from Alto Neuroscience, and equity from Mindstrong Health and Akili Interactive. S.H.S.: owner of intellectual property involving the use of brain connectivity to target TMS, scientific consultant for Magnus Medical, investigator-initiated research funding from Neuronetics and Brainsway, speaking fees from Brainsway and Otsuka (for PsychU.org), shareholder in Brainsway (publicly traded) and Magnus Medical (not publicly traded). None of these entities were directly involved in the present work. M.D.F.: scientific consultant for Magnus Medical, owner of independent intellectual property involving the use of functional connectivity to target TMS. This intellectual property was not used in the present manuscript.
Peer review
Peer review information
Nature Human Behaviour thanks Carissa Philippi, Ronny Redlich and Adrienne Romer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taylor, J.J., Lin, C., Talmasov, D. et al. A transdiagnostic network for psychiatric illness derived from atrophy and lesions. Nat Hum Behav 7, 420–429 (2023). https://doi.org/10.1038/s41562-022-01501-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-022-01501-9
This article is cited by
-
Dissecting the shared genetic landscape of anxiety, depression, and schizophrenia
Journal of Translational Medicine (2024)
-
Probing prefrontal-sgACC connectivity using TMS-induced heart–brain coupling
Nature Mental Health (2024)
-
Divergent suicidal symptomatic activations converge on somato-cognitive action network in depression
Molecular Psychiatry (2024)
-
The future of brain circuit-targeted therapeutics
Neuropsychopharmacology (2024)
-
Closing the loop between brain and electrical stimulation: towards precision neuromodulation treatments
Translational Psychiatry (2023)