Abstract
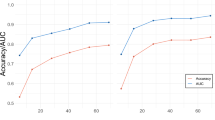
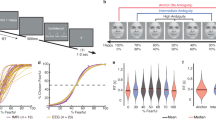
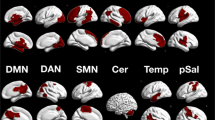
Functional neuroimaging techniques have been widely used to probe the neural substrates of facial emotion processing in healthy people. However, findings are largely inconsistent across studies. Here, we introduce a new technique termed activation network mapping to examine whether heterogeneous functional magnetic resonance imaging findings localize to a common network for emotion processing. First, using the existing method of activation likelihood estimation meta-analysis, we showed that individual-brain-based reproducibility was low across studies. Second, using activation network mapping, we found that network-based reproducibility across these same studies was higher. Validation analysis indicated that the activation network mapping-localized network aligned with stimulation sites, structural abnormalities and brain lesions that disrupt facial emotion processing. Finally, we verified the generality of the activation network mapping technique by applying it to another cognitive process, that is, rumination. Activation network mapping may potentially be broadly applicable to localize brain networks of cognitive functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The normative connectome datasets are publicly available from the Human Connectome Project (HCP, https://www.humanconnectome.org) and the Genome Superstruct Project (GSP); The coordinate information for both the facial emotion processing and non-emotional processing, as well as the raw images for all figures in the main text and Supplementary Information, are publicly available at https://github.com/sailingpeng/2021_ActivationNetworkMapping.git.
Code availability
Custom codes for ANM analysis are publicly available on GitHub (https://github.com/sailingpeng/2021_ActivationNetworkMapping.git).
References
Hamann, S. Mapping discrete and dimensional emotions onto the brain: controversies and consensus. Trends Cogn. Sci. 16, 458–466 (2012).
Ekman, P. An argument for basic emotions. Cogn. Emot. 6, 169–200 (1992).
Wilson-Mendenhall, C. D., Barrett, L. F., Simmons, W. K. & Barsalou, L. W. Grounding emotion in situated conceptualization. Neuropsychologia 49, 1105–1127 (2011).
Simmons, J. P., Nelson, L. D. & Simonsohn, U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol. Sci. 22, 1359–1366 (2011).
Ioannidis, J. P. Why most published research findings are false. PLoS Med. 2, e124 (2005).
Eickhoff, S. B. et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926 (2009).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Bressler, S. L. & Menon, V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14, 277–290 (2010).
Darby, R. R., Joutsa, J. & Fox, M. D. Network localization of heterogeneous neuroimaging findings. Brain 142, 70–79 (2019).
Boes, A. D. et al. Network localization of neurological symptoms from focal brain lesions. Brain 138, 3061–3075 (2015).
Fasano, A., Laganiere, S. E., Lam, S. & Fox, M. D. Lesions causing freezing of gait localize to a cerebellar functional network. Ann. Neurol. 81, 129–141 (2017).
Laganiere, S., Boes, A. D. & Fox, M. D. Network localization of hemichorea-hemiballismus. Neurology 86, 2187–2195 (2016).
Albazron, F. M. et al. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology 93, e1561–e1571 (2019).
Klingbeil, J., Wawrzyniak, M., Stockert, A., Karnath, H. O. & Saur, D. Hippocampal diaschisis contributes to anosognosia for hemiplegia: evidence from lesion network-symptom-mapping. NeuroImage 208, 116485 (2020).
Lee, I. et al. Diverse pathophysiological processes converge on network disruption in mania. J. Affect. Disord. 244, 115–123 (2019).
Sutterer, M. J. et al. Canceled connections: lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex 78, 31–43 (2016).
Lim, J. S. et al. Individual-level lesion-network mapping to visualize the effects of a stroke lesion on the brain network: connectograms in stroke syndromes. J. Clin. Neurol. 16, 116–123 (2020).
Philippi, C. L. et al. Lesion network mapping demonstrates that mind-wandering is associated with the default mode network. J. Neurosci. Res. 99, 361–373 (2021).
Wawrzyniak, M., Klingbeil, J., Zeller, D., Saur, D. & Classen, J. The neuronal network involved in self-attribution of an artificial hand: a lesion network-symptom-mapping study. NeuroImage 166, 317–324 (2018).
Mithani, K. et al. Lesion network localization of seizure freedom following MR-guided laser interstitial thermal ablation. Sci. Rep. 9, 18598 (2019).
Tetreault, A. M. et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain 143, 1249–1260 (2020).
Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 (2020).
Cole, M. W., Ito, T., Bassett, D. S. & Schultz, D. H. Activity flow over resting-state networks shapes cognitive task activations. Nat. Neurosci. 19, 1718–1726 (2016).
Tavor, I. et al. Task-free MRI predicts individual differences in brain activity during task performance. Science 352, 216–220 (2016).
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S. & Petersen, S. E. Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 (2014).
Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13040–13045 (2009).
Xu, P., Opmeer, E. M., van Tol, M. J., Goerlich, K. S. & Aleman, A. Structure of the alexithymic brain: a parametric coordinate-based meta-analysis. Neurosci. Biobehav. Rev. 87, 50–55 (2018).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Weil, R. S., Hsu, J. K., Darby, R. R., Soussand, L. & Fox, M. D. Neuroimaging in Parkinson’s disease dementia: connecting the dots. Brain Commun. 1, fcz006 (2019).
Button, K. S. et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376 (2013).
Lieberman, M. D. & Cunningham, W. A. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 4, 423–428 (2009).
Poldrack, R. A. et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017).
Sterne, J. A. & Davey Smith, G. Sifting the evidence-what’s wrong with significance tests? Br. Med. J. 322, 226–231 (2001).
Sperber, C. & Dadashi, A. The influence of sample size and arbitrary statistical thresholds in lesion-network mapping. Brain 143, e40 (2020).
Darby, R. R., Horn, A., Cushman, F. & Fox, M. D. Lesion network localization of criminal behavior. Proc. Natl Acad. Sci. USA 115, 601–606 (2018).
Pessoa, L. Reprint of: emotion and cognition and the amygdala: from ‘what is it?’ to ‘what’s to be done?’. Neuropsychologia 49, 681–694 (2011).
Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Ochsner, K. N. et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23, 483–499 (2004).
Lindquist, K. A., Wager, T. D., Kober, H., Bliss-Moreau, E. & Barrett, L. F. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 35, 121–143 (2012).
Kragel, P. A., Reddan, M. C., LaBar, K. S. & Wager, T. D. Emotion schemas are embedded in the human visual system. Sci. Adv. 5, eaaw4358 (2019).
Duvernoy, H. The Human Brain: Surface, Blood Supply, and Three-Dimensional Anatomy (Springer Verlag, 1999).
Damasio, A. R., Tranel, D. & Damasio, H. Face agnosia and the neural substrates of memory. Annu. Rev. Neurosci. 13, 89–109 (1990).
Saxe, R. & Powell, L. J. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 17, 692–699 (2006).
Olson, I. R., Plotzker, A. & Ezzyat, Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731 (2007).
Vytal, K. & Hamann, S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 22, 2864–2885 (2010).
Kober, H. et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage 42, 998–1031 (2008).
Kragel, P. A. & LaBar, K. S. Decoding the nature of emotion in the brain. Trends Cogn. Sci. 20, 444–455 (2016).
Pessoa, L. Understanding emotion with brain networks. Curr. Opin. Behav. Sci. 19, 19–25 (2018).
Pessoa, L. Understanding brain networks and brain organization. Phys. Life Rev. 11, 400–435 (2014).
Roy, M., Shohamy, D. & Wager, T. D. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 16, 147–156 (2012).
Yeshurun, Y., Nguyen, M. & Hasson, U. The default mode network: where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 22, 181–192 (2021).
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R. & Eickhoff, S. B. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534 (2010).
Nieuwenhuys, R. The insular cortex: a review. Prog. Brain Res 195, 123–163 (2012).
Augustine, J. R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Brain Res. Rev. 22, 229–244 (1996).
Adolphs, R., Tranel, D., Damasio, H. & Damasio, A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672 (1994).
Adolphs, R. Social attention and the ventromedial prefrontal cortex. Brain 137, 1572–1574 (2014).
Boucher, O. et al. Social information processing following resection of the insular cortex. Neuropsychologia 71, 1–10 (2015).
Honey, C. J., Thivierge, J. P. & Sporns, O. Can structure predict function in the human brain? NeuroImage 52, 766–776 (2010).
Joliot, M. et al. AICHA: an atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods 254, 46–59 (2015).
Sehatpour, P. et al. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc. Natl Acad. Sci. USA 105, 4399–4404 (2008).
Sestieri, C., Corbetta, M., Romani, G. L. & Shulman, G. L. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 31, 4407–4420 (2011).
Fedorenko, E. & Thompson-Schill, S. L. Reworking the language network. Trends Cogn. Sci. 18, 120–126 (2014).
Ptak, R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist 18, 502–515 (2012).
Lee, K. H., Farrow, T. F., Spence, S. A. & Woodruff, P. W. Social cognition, brain networks and schizophrenia. Psychol. Med. 34, 391–400 (2004).
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F. & Fox, P. T. Activation likelihood estimation meta-analysis revisited. NeuroImage 59, 2349–2361 (2012).
Lancaster, J. L. et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 28, 1194–1205 (2007).
Van Essen, D. C. et al. The WU-Minn human connectome project: an overview. NeuroImage 80, 62–79 (2013).
Karnath, H. O., Sperber, C. & Rorden, C. Mapping human brain lesions and their functional consequences. NeuroImage 165, 180–189 (2018).
Niazy, R. K., Xie, J., Miller, K., Beckmann, C. F. & Smith, S. M. Spectral characteristics of resting state networks. Prog. Brain Res 193, 259–276 (2011).
Sala-Llonch, R., Smith, S. M., Woolrich, M. & Duff, E. P. Spatial parcellations, spectral filtering, and connectivity measures in fMRI: optimizing for discrimination. Hum. Brain Mapp. 40, 407–419 (2019).
Holmes, A. J. et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci. Data 2, 150031 (2015).
Laird, A. R. et al. Networks of task co-activations. NeuroImage 80, 505–514 (2013).
Vuilleumier, P., Richardson, M. P., Armony, J. L., Driver, J. & Dolan, R. J. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat. Neurosci. 7, 1271–1278 (2004).
Wolf, R. C., Philippi, C. L., Motzkin, J. C., Baskaya, M. K. & Koenigs, M. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain 137, 1772–1780 (2014).
Barbey, A. K., Koenigs, M. & Grafman, J. Dorsolateral prefrontal contributions to human working memory. Cortex 49, 1195–1205 (2013).
Müller, N. G., Machado, L. & Knight, R. T. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J. Cogn. Neurosci. 14, 673–686 (2002).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289 (2002).
Zhou, H. X. et al. Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. NeuroImage 206, 116287 (2020).
Hamilton, J. P., Farmer, M., Fogelman, P. & Gotlib, I. H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry 78, 224–230 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 82172016 and 82021004 to G.G.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank M.D. Fox for valuable comments on this paper.
Author information
Authors and Affiliations
Contributions
S.P. and G.G. conceived the analysis. S.P. designed the study. S.P. collected the data and performed the analyses. Y.J. preprocessed the HCP and GSP datasets. S.P. wrote the manuscript. S.P., G.G. and P.X. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Nils Kohn, Alexander Cohen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Methods 1–6, Figs. 1–15, Tables 1–5 and references.
Rights and permissions
About this article
Cite this article
Peng, S., Xu, P., Jiang, Y. et al. Activation network mapping for integration of heterogeneous fMRI findings. Nat Hum Behav 6, 1417–1429 (2022). https://doi.org/10.1038/s41562-022-01371-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-022-01371-1
This article is cited by
-
Divergent suicidal symptomatic activations converge on somato-cognitive action network in depression
Molecular Psychiatry (2024)
-
Heterogeneous neuroimaging findings across substance use disorders localize to a common brain network
Nature Mental Health (2023)
-
Language abnormalities in schizophrenia: binding core symptoms through contemporary empirical evidence
Schizophrenia (2022)