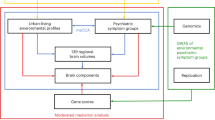
Abstract
Urbanicity is a growing environmental challenge for mental health. Here, we investigate correlations of urbanicity with brain structure and function, neuropsychology and mental illness symptoms in young people from China and Europe (total n = 3,867). We developed a remote-sensing satellite measure (UrbanSat) to quantify population density at any point on Earth. UrbanSat estimates of urbanicity were correlated with brain volume, cortical surface area and brain network connectivity in the medial prefrontal cortex and cerebellum. UrbanSat was also associated with perspective-taking and depression symptoms, and this was mediated by neural variables. Urbanicity effects were greatest when urban exposure occurred in childhood for the cerebellum, and from childhood to adolescence for the prefrontal cortex. As UrbanSat can be generalized to different geographies, it may enable assessments of correlations of urbanicity with mental illness and resilience globally.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data are available from the authors upon reasonable request and with permissions of the CHIMGEN and IMAGEN consortia.
Code availability
Custom code that supports the findings of this study is available from the corresponding author upon request.
References
Prince, M. et al. No health without mental health. Lancet 370, 859–877 (2007).
van Os, J., Kenis, G. & Rutten, B. P. The environment and schizophrenia. Nature 468, 203 (2010).
Bick, J. & Nelson, C. A. Early adverse experiences and the developing brain. Neuropsychopharmacology 41, 177 (2016).
Bhugra, D., Ventriglio, A., Castaldelli-Maia, J., & McCay, L. Urban Mental Health (Oxford Univ. Press, 2019).
Vlahov, D. & Galea, S. Urbanization, urbanicity, and health. J. Urban Health 79, S1–S12 (2002).
Heilig, G. K. World Urbanization Prospects: The 2011 Revision (United Nations DESA, 2012).
2018 Revision of World Urbanization Prospects (United Nations, 2018).
Evans, G. W. The built environment and mental health. J. Urban Health 80, 536–555 (2003).
Melchiorri, M. et al. Unveiling 25 years of planetary urbanization with remote sensing: perspectives from the global human settlement layer. Remote Sens 10, 768 (2018).
Seltenrich, N. Remote-sensing applications for environmental health research. Environ. Health Perspect. 122, A268–A275 (2014).
Xu, Q. et al. CHIMGEN: a Chinese imaging genetics cohort to enhance cross-ethnic and cross-geographic brain research. Mol. Psychiatry 25, 517–529 (2020).
Schumann, G. et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiatry 15, 1128–1139 (2010).
Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl Acad. Sci. USA 100, 253–258 (2003).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Lamm, C., Batson, C. D. & Decety, J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J. Cogn. Neurosci. 19, 42–58 (2007).
Peen, J., Schoevers, R. A., Beekman, A. T. & Dekker, J. The current status of urban–rural differences in psychiatric disorders. Acta Psychiatr. Scand. 121, 84–93 (2010).
Weiser, M. et al. Social and cognitive functioning, urbanicity and risk for schizophrenia. Br. J. Psychiatry 191, 320–324 (2007).
Hiser, J. & Koenigs, M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol. Psychiatry 83, 638–647 (2018).
Henckens, M. J. et al. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage 105, 312–322 (2015).
Pujol, J. et al. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage 129, 175–184 (2016).
Haddad, L. et al. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr. Bull. 41, 115–122 (2015).
Schmahmann, J. D. & Sherman, J. C. The cerebellar cognitive affective syndrome. Brain 121, 561–579 (1998).
Bambico, F. R. et al. High frequency stimulation of the anterior vermis modulates behavioural response to chronic stress: involvement of the prefrontal cortex and dorsal raphe? Neurobiol. Dis. 116, 166–178 (2018).
Carta, I., Chen, C. H., Schott, A. L., Dorizan, S. & Khodakhah, K. Cerebellar modulation of the reward circuitry and social behavior. Science 363, 6424 (2019).
Middleton, F. A. & Strick, P. L. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 21, 700–712 (2001).
Shaw, P. et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 (2008).
Lenroot, R. K. & Giedd, J. N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729 (2006).
Tiemeier, H. et al. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage 49, 63–70 (2010).
Lederbogen, F. et al. City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501 (2011).
Price, C., Dalman, C., Zammit, S. & Kirkbride, J. B. Association of residential mobility over the life course with nonaffective psychosis in 1.4 million young people in Sweden. JAMA Psychiatry 75, 1128–1136 (2018).
Fuhrmann, D., Knoll, L. J. & Blakemore, S. J. Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 19, 558–566 (2015).
Tang, Y. et al. Brain structure differences between Chinese and Caucasian cohorts: a comprehensive morphometry study. Hum. Brain Mapp. 39, 2147–2155 (2018).
Bremner, J. D. Stress and brain atrophy. CNS Neurol. Disord. Drug Targets 5, 503–512 (2006).
Guxens, M. et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatry 84, 295–303 (2018).
Khan, A. et al. Environmental pollution is associated with increased risk of psychiatric disorders in the US and Denmark. PLoS Biol. 17, e3000353 (2019).
Tyborowska, A. et al. Early-life and pubertal stress differentially modulate grey matter development in human adolescents. Sci. Rep. 8, 9201 (2018).
Paus, T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 9, 60–68 (2005).
Callaghan, B. L. & Tottenham, N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 7, 76–81 (2016).
Fonken, L. K. et al. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry 16, 987–995 (2011).
Elvidge, C. D. et al. Radiance calibration of DMSP-OLS low-light imaging data of human settlements. Remote Sens. Environ. 68, 77–88 (1999).
Liu, Z. et al. Extracting the dynamics of urban expansion in China using DMSP-OLS nighttime light data from 1992 to 2008. Landsc. Urban Plan. 106, 62–72 (2012).
Ma, T., Zhou, C., Pei, T., Haynie, S. & Fan, J. J. R. S. O. E. Quantitative estimation of urbanization dynamics using time series of DMSP/OLS nighttime light data: a comparative case study from China’s cities. Remote Sens. Environ. 124, 99–107 (2012).
Ghosh, T. et al. Shedding light on the global distribution of economic activity. Open Geogr. J. 3, 147–160 (2010).
Helbich, M. J. Spatiotemporal contextual uncertainties in green space exposure measures: exploring a time series of the normalized difference vegetation indices. Int. J. Environ. Res. Public Health 16, 852 (2019).
Zhou, Y. et al. A global map of urban extent from nightlights. Environ. Res. Lett. 10, 054011 (2015).
Esau, I. et al. Trends in normalized difference vegetation index (NDVI) associated with urban development in northern West Siberia. Atmos. Chem. Phys. 16, 9563–9577 (2016).
Zha, Y., Gao, J. & Ni, S. J. Use of normalized difference built-up index in automatically mapping urban areas from TM imagery. Int. J. Remote Sens. 24, 583–594 (2003).
McFeeters, S. K. The use of the normalized difference water index (NDWI) in the delineation of open water features. Int. J. Remote Sens. 17, 1425–1432 (1996).
Foley, J. A. et al. Global consequences of land use. Science 309, 570–574 (2005).
Gong, P. et al. Finer resolution observation and monitoring of global land cover: first mapping results with Landsat TM and ETM+ data. Int. J. Remote Sens. 34, 2607–2654 (2013).
Liu, X. et al. Identifying patterns and hotspots of global land cover transitions using the ESA CCI Land Cover dataset. Remote Sens. Lett. 9, 972–981 (2018).
Ashburner, J. & Ridgway, G. R. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front. Neurosci. 6, 197 (2013).
Buuren, S. V. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2010).
Rosseel, Y. Lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Hoyle, R. H. Structural Equation Modeling: Concepts, Issues, and Applications (Sage, 1995).
Raykov, T. Bias-corrected estimation of noncentrality parameters of covariance structure models. Struct. Equ. Modeling 12, 120–129 (2005).
Brown, T. A. Confirmatory Factor Analysis for Applied Research (Guilford, 2014).
Rubin, D. B. Multiple Imputation for Nonresponse in Surveys (Wiley, 2004).
Mollink, J. et al. The spatial correspondence and genetic influence of interhemispheric connectivity with white matter microstructure. Nat. Neurosci. 22, 809 (2019).
Viechtbauer, W. Conducting meta-analysis in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Gasparrini, A. J. Distributed lag linear and non-linear models in R: the package dlnm. J. Stat. Softw. 43, 1 (2011).
Preacher, K. J. & Hayes, A. F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res Methods 40, 879–891 (2008).
Hayes, A. F. Introduction to Mediation, Moderation, and Conditional Process Analysis: a Regression-Based Approach (Guilford, 2013).
Acknowledgements
This work was partly supported by the National Key Research and Development Program of China (grant no. 2018YFC1314301 to C.Y.), National Natural Science Foundation of China (grant no. 82001797 to J.X., 82030053 to C.Y., 81801687 to Q. Li and 81701668 to H.L.), National Key Research and Development Program of China (grant no. 2017YFA0604401 to L.Y. and 2019YFA0606601 to L.Y.), National Key Scientific and Technological Infrastructure project ‘Earth System Science Numerical Simulator Facility’ (EarthLab), European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT-2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), Human Brain Project (HBP SGA 2, 785907 and HBP SGA 3, 945539), Medical Research Council Grant ‘c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), National Institute of Health (NIH) (R01DA049238, A decentralized macro and micro gene-by-environment interaction analysis of substance use behaviour and its brain biomarkers), National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B), Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940, TRR 265, NE 1383/14-1), Medical Research Foundation and Medical Research Council (grants MR/R00465X/1 and MR/S020306/1) and National Institutes of Health (NIH) funded ENIGMA (grants 5U54EB020403-05 and 1R56AG058854-01). Further support was provided by grants from the ANR (ANR-12-SAMA-0004, AAPG2019-GeBra), Eranet Neuron (AF12-NEUR0008-01-WM2NA; and ANR-18-NEUR00002-01- ADORe), Fondation de France (00081242), Fondation pour la Recherche Médicale (DPA20140629802), Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, Fondation de l’Avenir (grant AP-RM-17-013), Fédération pour la Recherche sur le Cerveau; National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence and The Science&Technology Development Fund of Tianjin Education Commission for Higher Education (grant no. 2019KJ195 to J.X.). Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC-2049 – 390688087 Falls mehrere Projekte genannt werden müssen, könnte man das z.B. so gestalten: This work was supported by the Deutsche Forschungsgemeinschaft (Collaborative Research Grant SFB 958 (to DS (A5), SJS (A3, A6), CR (A5)) and Excellence Strategy’ – EXC-2049––390688087 (to DS, SJS, and CR). The authors thank C. Li, Department of Health Statistics, College of Public Health, Tianjin Medical University for statistical advice. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
G.S., C.Y. and J.X. designed the study. J.X., C.Y. and G.S. wrote the manuscript. All authors critically reviewed the manuscript. X.L., Q. Li, R.G., W.Q., F.L., C.C., Q. Luo, A.I., L.G., N.L., H.L., C.H., K.P., V.C., M.J.L., M.L., P.G., E.D.B., N.C., A.M., L.Y., C.Y. and G.S. were the principal investigators. J.C., M.W., Z.G., W.Z., B.Z., W.L., S.Q., H.Z., X.X., Y.Y., B.G., T.H., G.C., F.C., J.X., J.L., J.Z., X.Z., D.W., W.S., Y.M., F.Y., S.L., X.Z., K.X., L.Z., Z.Y., T.B., G.B., A.L.W.B., H.F., A.G., H.G., P.G., A.H., R.B., J.M., E.A., F.N., D.P.O., H. L., T.P., L.P., L.R., S.H., J.H.F., M.N.S., H.W., R.W. and J.W. acquired the data. A.M. supervised the statistical analysis. J.X. and Q. Li analysed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Human Behaviour thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 A flow diagram of sample selection in CHIMGEN (a) and IMAGEN (b).
BDI, Beck depression inventory; BTG, ball tossing games task; BL, IMAGEN baseline assessment acquired at 14 years; CIDI-AS, Anxiety Screening from the Composite International Diagnostic Interview; CVLT-II, the second edition of California verbal learning test; DAWBA-GA, Generalized Anxiety Scale from The Development and Well-Being Assessment Interview; FC, functional connectivity; FU2, IMAGEN second follow up assessment acquired at 19 years; FU2-BL, IMAGEN FU2-BL measures brain changes rate between BL of 14 years and FU2 of 19 years; GNG, go/no-go task; IRI, Interpersonal Reactivity Index; RSQ, Ruminating Scale Questionnaire; SA, state anxiety; SBM, surface-based morphometry; SDMT, symbol digit modalities test; TA, trait anxiety; TBSS, tract-based spatial statistics; VBM, voxel-based morphometry.
Extended Data Fig. 2 Schematic summary of multiple imputation and confirmatory factor analysis.
a. A flow diagram for multiple imputation and confirmatory factor analysis. b, c. Sensitivity analysis results in voxel-wise correlations of ten imputed UrbanSat (b) and combined UrbanSat (c) with brain GMV in CHIMGEN (FWE Pc<0.05). Each imputed UrbanSat from MICE imputation before 18 years show a significant negative correlation with left mPFC volume and a significant positive correlation with cerebellar volume adjusting confounding covariates (FWE Pc<0.05) (b), similar to the results derived from combined UrbanSat score following Rubin’s rule (c). d. The estimated fractions of missing information (FMI) of UrbanSat were low for the GMVs of left-mPFC-ROI (FMI = 1.01%) and cerebellum-ROI (FMI = 1.19%). UrbanSat was still correlated with mPFC-GMV (P < 0.001) and cerebellum-GMV (P < 0.001) after pooling using mice R package in CHIMGEN. e. Non-imputed mean UrbanSat before 18 years still show a significant negative correlation with mPFC-GMV and a significant positive correlation with cerebellar-GMV adjusting confounding covariates (FWE Pc<0.05) (n = 1460). CFA, confirmatory factor analysis; FMI, fractions of missing information; L, left; MICE, multivariate imputation by chained equations; mPFC, medial prefrontal cortex; R, right. S, satellite; Y, years.
Extended Data Fig. 3 Histograms of UrbanSat in each center of CHIMGEN (a) and IMAGEN (b).
TMUGH, Tianjin Medical University General Hospital; TMUCIH, Tianjin Medical University Cancer Institute and Hospital; TFCH, Tianjin First Center Hospital; CPAPFLUPH, Pingjin Hospital, Logistics University of Chinese People’s Armed Police Forces; THH, Tianjin Huanhu Hospital; HMUSH, The Second Hospital of Hebei Medical University; SMUFH, The First Hospital of Shanxi Medical University; DMUFAH, The First Affiliated Hospital of Dalian Medical University; NMUDTH, Drum Tower Hospital, Medical School of Nanjing University; XMUAH, The Affiliated Hospital of Xuzhou Medical University; ZUSAH, The Second Affiliated Hospital of Zhejiang University; WMUFAH, The First Affiliated Hospital of Wenzhou Medical University; WMUSAH, The Second Affiliated Hospital of Wenzhou Medical University; AMUFAH, The First Affiliated Hospital of Anhui Medical University; USTC, University of Science and Technology of China; SUQH, Qilu Hospital of Shandong University; YYH, Yantai Yuhuangding Hospital; ZUPH/HPPH, Zhengzhou University People’s Hospital and Henan Provincial People’s Hospital; ZUFAH, The First Affiliated Hospital of Zhengzhou University; HUSTTH, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology; CSUXH, Xiangya Hospital, Central South University; GUCMFAH, The First Affiliated Hospital of Guangzhou University of Chinese Medicine; HGH, Hainan General Hospital; FMMUTH, Tangdu Hospital, the Military Medical University of PLA Airforce (Fourth Military Medical University); LUSH, Lanzhou University Second Hospital; SUWCH, West China Hospital of Sichuan University; ZUPH, Zhengzhou University People’s Hospital; NMUJH, Jinling Hospital, Medical School of Nanjing University.
Extended Data Fig. 4 Correlations of UrbanSat with brain GMV, SA and CT in CHIMGEN and IMAGEN.
a. Uncorrected correlation statistical maps of UrbanSat with brain GMV in CHIMGEN under non-parametric permutation testing (n = 2176). b. Correlations of UrbanSat with brain GMV in CHIMGEN under Pc<0.05 in TFCE-FWE using non-parametric permutation testing (n = 2176). c, d. Uncorrected correlation statistical maps of UrbanSat with brain GMV in CHIMGEN (c) and IMAGEN-FU2 (d) under parametric testing. e. The overlap results (yellow) in the voxel-wise correlation of mean UrbanSat before 18 years with brain GMV in CHIMGEN (red) and IMAGEN-FU2 (green) after controlling confounders (FWE Pc<0.05). f,g. Uncorrected vertex-wise correlation maps of UrbanSat with surface area (f) and cortical thickness (g) in CHIMGEN (n = 2164). h,i. The mPFC-ROI projected onto the volumetric map (h) and fsaverage surface in Freesurfer (i).
Extended Data Fig. 5 Voxel-wise correlations of individual satellite measures with brain GMV in CHIMGEN (n = 2176) (a-e) and IMAGEN (n = 415) (f-j).
a–e. In CHIMGEN, there are significant negative correlations of mean night-time light (a) and population density (e) with mPFC GMV and positive correlations with cerebellar GMV after controlling confounders (FWE, Pc<0.05); There are significant negative correlations of mean built-up with mPFC GMV (b) and of mean cropland with cerebellar GMV (c); There are no correlations of mean NDVI with brain GMV (d). f–j. In IMAGEN, there are significant negative correlations of mean night-time light (f), mean built-up (g) and population density (j) with mPFC GMV and positive correlations with cerebellar GMV after controlling confounders (FWE, Pc<0.05); There are no correlations of mean cropland (h) and NDVI (i) with brain GMV. L, left; mPFC, medial prefrontal cortex; NDVI, normalized difference vegetation index; R, right.
Extended Data Fig. 6 Forest plot of meta-analysis in CHIMGEN and IMAGEN-FU2.
Effect size of correlations of UrbanSat with mPFC GMV (a), cerebellar GMV (b), mPFC CT (c), mPFC SA (d), WNFCs in aDMN (e), CN (f), mVN (g) and lVN (h), BNFCs of aDMN-CN (i), aDMN-ECN (j), aDMN-rFPN (k) and rFPN-lFPN (l) for meta-analysis in CHIMGEN and IMAGEN-FU2. We exclude SUWCH center from CHIMGEN for all meta-analysis and Dublin center from IMAGEN for the meta-analysis of brain functional features, because there are only 8 and 10 participants from each site, which more than the numbers of covariates while performing Spearman correlation analysis.
Extended Data Fig. 7 Susceptibility analysis of individual satellite measures with brain (a-d) and behaviors (e-h) using distributed lag models in CHIMGEN.
a. There are significant associations of lifetime night-time light with the mPFC-ROI GMV (ages of 4–14 years) and SA (5–12 years), WNFC in aDMN (3–11 years) during childhood and adolescence, with cerebellum-ROI GMV (3–7 years), WNFCs in CN (0–6 years), mVN (0–6 years), lVN (3–10 years), BNFCs in aDMN-CN (4–7 years), aDMN-ECN (4–6 years), aDMN-rFPN (4–6 years) and rFPN-lFPN (4–6 years) during childhood in CHIMGEN. b. There are significant associations of lifetime built-up% with the mPFC-ROI GMV (5–16 years) and WNFC in aDMN (4–14 years) during childhood and adolescence, with WNFCs in mVN and lVN (14–20 years) during adolescence, with mPFC-ROI SA (5–7 years), cerebellum-ROI GMV (1–10 years), WNFC in CN (1–10 years), BNFCs in aDMN-CN (4–10 years), aDMN-ECN (5–7 years), aDMN-rFPN (4–10 years) and rFPN-lFPN (4–6 years) during childhood in CHIMGEN. c. There are significant associations of lifetime cropland% with the mPFC-ROI GMV (5–15 years) during childhood and adolescence, with mPFC-ROI SA (5–6 years), cerebellum-ROI GMV (4–6 years), WNFCs in aDMN (4–6 years), CN (4–6 years) and lVN (4–10 years), BNFCs in aDMN-CN (0–9 years), aDMN-ECN (2–7 years), aDMN-rFPN (4–10 years) and rFPN-lFPN (4–6 years) during childhood in CHIMGEN. d. We find significant associations of lifetime NDVI with the mPFC-ROI GMV (5–15 years) and BNFC in rFPN-lFPN (6–17 years) during childhood and adolescence, with WNFCs in aDMN (5 years old) and CN (5 years old), BNFCs in aDMN-CN (4–11 years) and aDMN-rFPN (4–10 years) during childhood in CHIMGEN. There are significant correlations of lifetime night-time light (e), built-up% (f), cropland % (g) and NDVI (h) with reaction time for perspective-taking performance during adolescence (ages of 5–16 years for night-time light, 4–17 years for built-up %, 5–19 years for cropland % and 4–17 years for NDVI) in CHIMGEN. Significant correlations of lifetime night-time light (e), built-up % (f), cropland % (g) and NDVI (h) with increasing depression measured by BDI are also observed during childhood in CHIMGEN (0–6 years for night-time light, 2–9 years for built-up %, 0–9 years for cropland % and 3–11 years for NDVI). The y-axis represents the changes of brain behaviors associated with an increase of interquartile range of individual satellite measures; the x-axis is individual satellite measure lag in ages. Grey areas indicate 95% CIs. A susceptibility window is identified for the ages where the estimated pointwise 95% CI (shaded area) does not include zero. The blue solid lines indicate negative correlations and red ones indicated positive correlations. aDMN, anterior default mode network; BDI, Beck depression index; BNFC, between-network functional connectivity; CN, cerebellar network; CT, cortical thickness; GMV, grey matter volume; lVN, lateral visual network; mPFC, medial prefrontal cortex; mVN, medial visual network; RTpt, reaction time for perspective-taking; SA, surface area; WNFC, within-network functional connectivity.
Extended Data Fig. 8 Seventeen RSNs identified by independent component analysis in CHIMGEN.
aDMN, anterior default mode network; AN, auditory network; aSN, anterior cingulate cortex part of salience network; CN, cerebellar network; dAN, dorsal attentional network; dSMN, dosal sensorimotor network; ECN, executive control network; inSN, insular part of salience network; lFPN, left frontal parietal network; LN, language network; lVN, lateral visual network; mVN, medial visual network; pDMN, posterior default mode network; PN, precuneus network; rFPN, right frontal parietal network; RSNs, resting-state networks; vAN, ventral attentional network; vSMN, ventral sensorimotor network.
Extended Data Fig. 9 Voxel-wise correlations of individual satellite measures with WNFCs and BNFCs in CHIMGEN (n = 2156) and IMAGEN (n = 315).
a–f. In CHIMGEN, there are negative correlations (blue) of mean UrbanSat (a) and mean night-time light before 18 years (b) with WNFC in the mPFC of the aDMN, positive correlations (red) with WNFCs in the left CV of the CN and left LG of the mVN and lVN (FWE Pc<0.05). c. There are negative correlations (blue) of mean built-up% before 18 years with WNFC in the mPFC of the aDMN and positive correlations (red) with WNFC in the left LG of the lVN (FWE Pc<0.05). d. There are negative correlations (blue) of mean cropland% before 18 years with WNFCs in the CV of the CN and the left LG of the lVN (FWE Pc<0.05). e. There is no correlation of mean NDVI with WNFC of any RSN surviving the multiple correction. f. There are negative correlations (blue) of mean population density from GHSL before 18 years with WNFC in the mPFC of the aDMN, positive correlations (red) with WNFCs in the left CV of the CN and the left LG of the mVN (FWE Pc<0.05). g–l. In IMAGEN, there are negative correlations (blue) of mean UrbanSat (g), night-time light (h), built-up% (i) before 18 years with WNFC in the mPFC of the aDMN, positive correlations (red) with WNFC in the CV of the CN (FWE Pc<0.05). j. There are negative correlations (blue) of mean cropland before 18 years with WNFC in the CV of the CN (FWE Pc<0.05). k. There is no correlation of mean NDVI with WNFC of any RSN surviving the multiple correction. l. There are negative correlations (blue) of mean population density from GHSL before 18 years with WNFC in the mPFC of the aDMN, and positive correlation (red) with WNFC in the CV of the CN (FWE, Pc<0.05). m–p. The mean built-up% (m) (N = 32), cropland% (n) (N = 41), NDVI (o) (N = 1) and population density (p) (N = 52) show correlations with BNFCs in CHIMGEN. The red line indicates positive correlations of UrbanSat with BNFCs and blue line indicated negative correlations. N indicates the numbers of significant correlations of BNFCs. aDMN, anterior default mode network; CV, cerebellar vermis; CN, cerebellar network; GHSL, global human settlement layers; LG, lingual gyrus; lVN, lateral visual network; mPFC, medial prefrontal cortex; mVN, medial visual network; NDVI, normalized difference vegetation index; WNFC, within-network functional connectivity.
Extended Data Fig. 10 The schematic summary of ball tossing game task design, which measures perspective taking and agency performance.
ACT, active agency; 1PP, first-person perspective; 3PP, third-person perspective; PAS, passive agency.
Supplementary information
Supplementary information
Supplementary Methods, Results, Tables 1–24 and References.
Rights and permissions
About this article
Cite this article
Xu, J., Liu, X., Li, Q. et al. Global urbanicity is associated with brain and behaviour in young people. Nat Hum Behav 6, 279–293 (2022). https://doi.org/10.1038/s41562-021-01204-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-021-01204-7
This article is cited by
-
How does the macroenvironment influence brain and behaviour—a review of current status and future perspectives
Molecular Psychiatry (2024)
-
Neural Correlates of Early-Life Urbanization and Their Spatial Relationships with Gene Expression, Neurotransmitter, and Behavioral Domain Atlases
Molecular Neurobiology (2024)
-
The effects of environmental factors associated with childhood urbanicity on brain structure and cognition
BMC Psychiatry (2023)
-
Increasing diversity in connectomics with the Chinese Human Connectome Project
Nature Neuroscience (2023)
-
Challenges and future directions for investigating the effects of urbanicity on mental health
Nature Mental Health (2023)