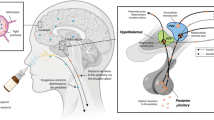
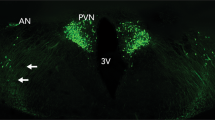
Abstract
The neuropeptide oxytocin has been popularized for its role in social behaviour and nominated as a candidate treatment for several psychiatric illnesses due to promising preclinical results. However, these results so far have failed to reliably translate from animal models to human research. In response, there have been justified calls to improve intranasal oxytocin delivery methodology in terms of verifying that intranasal administration increases central levels of oxytocin. Nonetheless, improved methodology needs to be coupled with a robust theory of the role of oxytocin in behaviour and physiology to ask meaningful research questions. Moreover, stringent methodology based on robust theory may yield interesting results, but such findings will have limited utility if they are not reproducible. We outline how the precision of intranasal oxytocin research can be improved by the complementary consideration of methodology, theory and reproducibility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Guastella, A. J. & MacLeod, C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm. Behav. 61, 410–418 (2012).
Yatawara, C. J., Einfeld, S. L., Hickie, I. B., Davenport, T. A. & Guastella, A. J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol. Psychiatry 21, 1225–1231 (2016).
Peled-Avron, L., Abu-Akel, A. & Shamay-Tsoory, S. Exogenous effects of oxytocin in five psychiatric disorders: a systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neurosci. Biobehav. Rev. 114, 70–95 (2020).
Insel, T. R. & Shapiro, L. E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. USA 89, 5981–5985 (1992).
Olazábal, D. E. & Young, L. J. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141, 559–568 (2006).
Schafer, E. A. & Mackenzie, K. The action of animal extracts on milk secretion. Proc. R. Soc. Lond., B 84, 16–22 (1911).
Dale, H. H. On some physiological actions of ergot. J. Physiol. (Lond.) 34, 163–206 (1906).
Coghlan, A. ‘Cuddle chemical’ eases symptoms of schizophrenia. New Sci. 207, 10 (2010).
Shamay-Tsoory, S. G. et al. Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biol. Psychiatry 66, 864–870 (2009).
Dadds, M. R. et al. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J. Autism Dev. Disord. 44, 521–531 (2014).
O’Leary, D. E. Gartner’s hype cycle and information system research issues. Int. J. Account. Inf. Syst. 9, 240–252 (2008).
Alvares, G. A., Quintana, D. S. & Whitehouse, A. J. O. Beyond the hype and hope: critical considerations for intranasal oxytocin research in autism spectrum disorder. Autism Res. 10, 25–41 (2017).
Leng, G. & Ludwig, M. Intranasal oxytocin: myths and delusions. Biol. Psychiatry 79, 243–250 (2016).
Gwee, P.-C., Tay, B.-H., Brenner, S. & Venkatesh, B. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol. 9, 47 (2009).
Stafflinger, E. et al. Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. Proc. Natl. Acad. Sci. USA 105, 3262–3267 (2008).
Auyeung, B. et al. Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl. Psychiatry 5, e507 (2015).
Guastella, A. J. et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry 56, 444–452 (2015).
Buchanan, R. W. et al. A randomized clinical trial of oxytocin or galantamine for the treatment of negative symptoms and cognitive impairments in people with schizophrenia. J. Clin. Psychopharmacol. 37, 394–400 (2017).
Davis, M. C. et al. Oxytocin-augmented social cognitive skills training in schizophrenia. Neuropsychopharmacology 39, 2070–2077 (2014).
Walum, H., Waldman, I. D. & Young, L. J. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry 79, 251–257 (2016).
Muthukrishna, M. & Henrich, J. A problem in theory. Nat. Hum. Behav. 3, 221–229 (2019).
Klopfer, P. H. & Klopfer, M. S. Maternal “imprinting” in goats: fostering of alien young. Ethology 25, 862–866 (1968).
Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U. & Fehr, E. Oxytocin increases trust in humans. Nature 435, 673–676 (2005).
Nave, G., Camerer, C. & McCullough, M. Does oxytocin increase trust in humans? A critical review of research. Perspect. Psychol. Sci. 10, 772–789 (2015).
Declerck, C. H., Boone, C., Pauwels, L., Vogt, B. & Fehr, E. A registered replication study on oxytocin and trust. Nat. Hum. Behav. 4, 646–655 (2020).
Shamay-Tsoory, S. G. & Abu-Akel, A. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202 (2016).
Kemp, A. H. & Guastella, A. J. The role of oxytocin in human affect a novel hypothesis. Curr. Dir. Psychol. Sci. 20, 222–231 (2011).
Quintana, D. S. & Guastella, A. J. An allostatic theory of oxytocin. Trends Cogn. Sci. 24, 515–528 (2020).
Harari-Dahan, O. & Bernstein, A. A general approach-avoidance hypothesis of oxytocin: accounting for social and non-social effects of oxytocin. Neurosci. Biobehav. Rev. 47, 506–519 (2014).
Quintana, D. S. et al. Low-dose intranasal oxytocin delivered with breath powered device modulates pupil diameter and amygdala activity: a randomized controlled pupillometry and fMRI study. Neuropsychopharmacology 44, 306–313 (2019).
Harari-Dahan, O. & Bernstein, A. Oxytocin attenuates social and non-social avoidance: re-thinking the social specificity of oxytocin. Psychoneuroendocrinology 81, 105–112 (2017).
Fam, J., Holmes, N., Delaney, A., Crane, J. & Westbrook, R. F. Oxytocin receptor activation in the basolateral complex of the amygdala enhances discrimination between discrete cues and promotes configural processing of cues. Psychoneuroendocrinology 96, 84–92 (2018).
Zhao, Z. et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 184, 781–789 (2019).
Eliava, M. et al. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89, 1291–1304 (2016).
Wang, Y.-L. et al. The interaction between the oxytocin and pain modulation in headache patients. Neuropeptides 47, 93–97 (2013).
Boll, S., Almeida de Minas, A. C., Raftogianni, A., Herpertz, S. C. & Grinevich, V. Oxytocin and pain perception: from animal models to human research. Neuroscience 387, 149–161 (2018).
Insel, T. R. Translating oxytocin neuroscience to the clinic: a national institute of mental health perspective. Biol. Psychiatry 79, 153–154 (2016).
King, L. B., Walum, H., Inoue, K., Eyrich, N. W. & Young, L. J. Variation in the oxytocin receptor gene predicts brain region–specific expression and social attachment. Biol. Psychiatry 80, 160–169 (2016).
Keebaugh, A. C. & Young, L. J. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav. 60, 498–504 (2011).
Loup, F., Tribollet, E., Dubois-Dauphin, M. & Dreifuss, J. J. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 555, 220–232 (1991).
Uhrig, S. et al. Reduced oxytocin receptor gene expression and binding sites in different brain regions in schizophrenia: A post-mortem study. Schizophr. Res. 177, 59–66 (2016).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Quintana, D. S. et al. Oxytocin pathway gene networks in the human brain. Nat. Commun. 10, 668 (2019).
Paloyelis, Y. et al. A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biol. Psychiatry 79, 693–705 (2016).
Beard, R., Singh, N., Grundschober, C., Gee, A. D. & Tate, E. W. High-yielding 18F radiosynthesis of a novel oxytocin receptor tracer, a probe for nose-to-brain oxytocin uptake in vivo. Chem. Commun. (Camb.) 54, 8120–8123 (2018).
Love, T. M. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 119, 49–60 (2014).
Feldman, R., Feldman, R., Monakhov, M., Pratt, M. & Ebstein, R. P. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol. Psychiatry 79, 174–184 (2015).
Gimpl, G. & Fahrenholz, F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81, 629–683 (2001).
Leng, G. & Sabatier, N. Oxytocin – the sweet hormone? Trends Endocrinol. Metab. 28, 365–376 (2017).
Stanley, S. A. et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature 531, 647–650 (2016).
Noble, E. E., Billington, C. J., Kotz, C. M. & Wang, C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R737–R745 (2014).
Geerling, J. C., Shin, J. W., Chimenti, P. C. & Loewy, A. D. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J. Comp. Neurol. 518, 1460–1499 (2010).
Browning, K. N. & Travagli, R. A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 4, 1339–1368 (2014).
Maejima, Y. et al. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 3, 1169–1177 (2011).
Sabatier, N., Leng, G. & Menzies, J. Oxytocin, feeding, and satiety. Front. Endocrinol. (Lausanne) 4, 35 (2013).
Lawson, E. A. et al. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 23, 950–956 (2015).
Ott, V. et al. Oxytocin reduces reward-driven food intake in humans. Diabetes 62, 3418–3425 (2013).
Zhang, H. et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 8, e61477 (2013).
Valentino, R. J., Foote, S. L. & Page, M. E. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann. NY Acad. Sci. 697, 173–188 (1993).
Grewen, K. M. & Light, K. C. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol. Psychol. 87, 340–349 (2011).
Tops, M., van Peer, J. M., Korf, J., Wijers, A. A. & Tucker, D. M. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology 44, 444–449 (2007).
Nicolson, N. A., Davis, M. C., Kruszewski, D. & Zautra, A. J. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosom. Med. 72, 471–480 (2010).
Norman, G. J. et al. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol. Psychol. 86, 174–180 (2011).
Gutkowska, J., Jankowski, M., Mukaddam-Daher, S. & McCann, S. M. Oxytocin is a cardiovascular hormone. Braz. J. Med. Biol. Res. 33, 625–633 (2000).
Porges, S.W. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-regulation. (W. W. Norton & Co., 2011).
Akselrod, S. et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213, 220–222 (1981).
Quintana, D. S., Guastella, A. J., Outhred, T., Hickie, I. B. & Kemp, A. H. Heart rate variability is associated with emotion recognition: direct evidence for a relationship between the autonomic nervous system and social cognition. Int. J. Psychophysiol. 86, 168–172 (2012).
Butler, E. A., Wilhelm, F. H. & Gross, J. J. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology 43, 612–622 (2006).
Berntson, G. G., Cacioppo, J. T. & Grossman, P. Whither vagal tone. Biol. Psychol. 74, 295–300 (2007).
Kemp, A. H. et al. Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One 7, e44014 (2012).
Kubzansky, L. D., Mendes, W. B., Appleton, A. A., Block, J. & Adler, G. K. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biol. Psychol. 90, 1–9 (2012).
Everett, N.A., Turner, A.J., Costa, P.A., Baracz, S.J. & Cornish, J.L. The vagus nerve mediates the suppressing effects of peripherally administered oxytocin on methamphetamine self-administration and seeking in rats. Neuropsychopharmacology https://doi.org/10.1038/s41386-020-0719-7 (2020).
Neumann, I. D. & Landgraf, R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659 (2012).
Quintana, D. S., Alvares, G. A., Hickie, I. B. & Guastella, A. J. Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neurosci. Biobehav. Rev. 49, 182–192 (2015).
Li, Y., Field, P. M. & Raisman, G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia 52, 245–251 (2005).
Thorne, R. G., Emory, C. R., Ala, T. A. & Frey, W. H. II Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 692, 278–282 (1995).
Guastella, A. J. et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38, 612–625 (2013).
Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 9, 566–582 (2012).
Smith, A. S., Korgan, A. C. & Young, W. S. Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol. Res. 146, 104324 (2019).
Striepens, N. et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 3, 3440 (2013).
Dal Monte, O., Noble, P. L., Turchi, J., Cummins, A. & Averbeck, B. B. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One 9, e103677 (2014).
Lee, M. R. et al. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol. Psychiatry 23, 115–122 (2018).
Quintana, D. S. et al. Low-dose oxytocin delivered intranasally with breath powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Transl. Psychiatry 5, e602 (2015).
Hollander, E. et al. Oxytocin increases retention of social cognition in autism. Biol. Psychiatry 61, 498–503 (2007).
Hollander, E. et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology 28, 193–198 (2003).
Neumann, I. D., Maloumby, R., Beiderbeck, D. I., Lukas, M. & Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38, 1985–1993 (2013).
Quintana, D. S. et al. Low dose intranasal oxytocin delivered with breath powered device dampens amygdala response to emotional stimuli: A peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology 69, 180–188 (2016).
Lee, M. R. et al. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat. Commun. 11, 2783 (2020).
van der Aart, J. et al. First in human evaluation of [18F]PK-209, a PET ligand for the ion channel binding site of NMDA receptors. EJNMMI Res. 8, 69 (2018).
MacLean, E. L. et al. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology 107, 225–231 (2019).
Quintana, D. S. et al. Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm. Behav. 102, 85–92 (2018).
Valstad, M. et al. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 78, 117–124 (2017).
Brandtzaeg, O. K. et al. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci. Rep. 6, 31693 (2016).
Leng, G. & Sabatier, N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol. https://doi.org/10.1111/jne.12413 (2016).
Lefevre, A. et al. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci. Rep. 7, 17222 (2017).
Jurek, B. & Neumann, I. D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 98, 1805–1908 (2018).
Quintana, D. S., Guastella, A. J., Westlye, L. T. & Andreassen, O. A. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol. Psychiatry 21, 29–38 (2016).
Baker, M. 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454 (2016).
Quintana, D. Most oxytocin administration studies are statistically underpowered to reliably detect (or reject) a wide range of effect sizes. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/kzp4n (2020).
Mierop, A. et al. How can intranasal oxytocin research be trusted? A systematic review of the interactive effects of intranasal oxytocin on psychosocial outcomes. Perspect. Psychol. Sci. 15, 1228–1242 (2020).
Munafò, M. R. et al. A manifesto for reproducible science. Nat. Hum. Behav. 1, 0021 (2017).
Morey, R. & Lakens, D. Why most of psychology is statistically unfalsifiable. Preprint at Zenodo https://doi.org/10.5281/zenodo.838684 (2016).
Dienes, Z. Using Bayes to get the most out of non-significant results. Front. Psychol. 5, 781 (2014).
Hauck, W. W. & Anderson, S. A new statistical procedure for testing equivalence in two-group comparative bioavailability trials. J. Pharmacokinet. Biopharm. 12, 83–91 (1984).
Lakens, D. Equivalence tests: a practical primer for t tests, correlations, and meta-analyses. Soc. Psychol. Personal. Sci. 8, 355–362 (2017).
Wagenmakers, E.-J. et al. Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon. Bull. Rev. 25, 35–57 (2018).
Quintana, D. S. & Williams, D. R. Bayesian alternatives for common null-hypothesis significance tests in psychiatry: a non-technical guide using JASP. BMC Psychiatry 18, 178 (2018).
Quintana, D. S. Revisiting non-significant effects of intranasal oxytocin using equivalence testing. Psychoneuroendocrinology 87, 127–130 (2018).
Tabak, B. A. et al. Null results of oxytocin and vasopressin administration across a range of social cognitive and behavioral paradigms: Evidence from a randomized controlled trial. Psychoneuroendocrinology 107, 124–132 (2019).
Mello, M. M., Lieou, V. & Goodman, S. N. Clinical trial participants’ views of the risks and benefits of data sharing. N. Engl. J. Med. 378, 2202–2211 (2018).
Reiter, J. P. Releasing multiply imputed, synthetic public use microdata: an illustration and empirical study. J. R. Stat. Soc. Ser. A Stat. Soc. 168, 185–205 (2005).
Quintana, D. S. A synthetic dataset primer for the biobehavioural sciences to promote reproducibility and hypothesis generation. eLife 9, e53275 (2020).
Lane, A., Luminet, O., Nave, G. & Mikolajczak, M. Is there a publication bias in behavioural intranasal oxytocin research on humans? Opening the file drawer of one laboratory. J. Neuroendocrinol. https://doi.org/10.1111/jne.12384 (2016).
McCullough, M. E., Churchland, P. S. & Mendez, A. J. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 37, 1485–1492 (2013).
Marín, O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat. Med. 22, 1229–1238 (2016).
Kamesh, N., Aradhyam, G. K. & Manoj, N. The repertoire of G protein-coupled receptors in the sea squirt Ciona intestinalis. BMC Evol. Biol. 8, 129 (2008).
Ding, C., Leow, M. K.-S. & Magkos, F. Oxytocin in metabolic homeostasis: implications for obesity and diabetes management. Obes. Rev. 20, 22–40 (2019).
Palkovits, M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Geoffrey Harris Memorial Lecture, Kitakyushu, Japan, October 1998. Front. Neuroendocrinol. 20, 270–295 (1999).
Tamma, R. et al. Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. USA 106, 7149–7154 (2009).
Colaianni, G. et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem. Biophys. Res. Commun. 411, 512–515 (2011).
Nesse, R. M. Tinbergen’s four questions: two proximate, two evolutionary. Evol. Med. Public Health 2019, 2 (2018).
Fujino, Y. et al. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J. Exp. Zool. 284, 401–406 (1999).
Kawada, T., Sekiguchi, T., Itoh, Y., Ogasawara, M. & Satake, H. Characterization of a novel vasopressin/oxytocin superfamily peptide and its receptor from an ascidian, Ciona intestinalis. Peptides 29, 1672–1678 (2008).
Beets, I. et al. Vasopressin/oxytocin-related signaling regulates gustatory associative learning in C. elegans. Science 338, 543–545 (2012).
Chini, B., Leonzino, M., Braida, D. & Sala, M. Learning about oxytocin: pharmacologic and behavioral issues. Biol. Psychiatry 76, 360–366 (2014).
Wallis, M. Molecular evolution of the neurohypophysial hormone precursors in mammals: Comparative genomics reveals novel mammalian oxytocin and vasopressin analogues. Gen. Comp. Endocrinol. 179, 313–318 (2012).
Freeman, S.M. & Young, L.J. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol. https://doi.org/10.1111/jne.12382 (2016).
Carter, C. S. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav. Brain Res. 176, 170–186 (2007).
Holt-Lunstad, J., Birmingham, W. & Light, K. C. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology 36, 1249–1256 (2011).
Weisman, O., Zagoory-Sharon, O., Schneiderman, I., Gordon, I. & Feldman, R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology 38, 694–701 (2013).
Zhong, S. et al. U-shaped relation between plasma oxytocin levels and behavior in the trust game. PLoS One 7, e51095 (2012).
Geng, Y. et al. Oxytocin enhancement of emotional empathy: generalization across cultures and effects on amygdala activity. Front. Neurosci. 12, 512 (2018).
Feldman, R. et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry 72, 175–181 (2012).
Rilling, J. K. et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237–248 (2014).
Lieberz, J. et al. Kinetics of oxytocin effects on amygdala and striatal reactivity vary between women and men. Neuropsychopharmacology 45, 1134–1140 (2020).
Simonsohn, U., Nelson, L. D. & Simmons, J. P. P-curve and effect size: correcting for publication bias using only significant results. Perspect. Psychol. Sci. 9, 666–681 (2014).
Gilbert, C., Brown, M. C. J., Cappelleri, J. C., Carlsson, M. & McKenna, S. P. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest 135, 137–142 (2009).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
DeBruine, L. & Barr, D.J. Understanding mixed effects models through data simulation. Preprint at PsyArXiv https://doi.org/10.31234/osf.io/xp5cy (2019).
Lakens, D., Scheel, A. M. & Isager, P. M. Equivalence testing for psychological research: a tutorial. Adv. Methods Practices Psychol. Sci. 1, 259–269 (2018).
Grinevich, V., Desarménien, M. G., Chini, B., Tauber, M. & Muscatelli, F. Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front. Neuroanat. 8, 164 (2015).
Goudsmit, E., Neijmeijer-Leloux, A. & Swaab, D. F. The human hypothalamo-neurohypophyseal system in relation to development, aging and Alzheimer’s disease. Prog. Brain Res. 93, 237–247 (1992). discussion 247–248.
Hammock, E. A. D. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40, 24–42 (2015).
Vaidyanathan, R. & Hammock, E. A. D. Oxytocin receptor dynamics in the brain across development and species. Dev. Neurobiol. 77, 143–157 (2017).
Zheng, J.-J. et al. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat. Neurosci. 17, 391–399 (2014).
Winslow, J. T. et al. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm. Behav. 37, 145–155 (2000).
Alberts, J. R. Huddling by rat pups: ontogeny of individual and group behavior. Dev. Psychobiol. 49, 22–32 (2007).
Feldman, R. Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391 (2012).
Clark, C. L. et al. Neonatal CSF oxytocin levels are associated with parent report of infant soothability and sociability. Psychoneuroendocrinology 38, 1208–1212 (2013).
Acknowledgements
This work was supported by an Excellence Grant from the Novo Nordisk Foundation (NNF16OC0019856), the Research Council of Norway (301767) and the European Research Council under the European Union’s Horizon 2020 research and Innovation program (ERC StG, Grant 802998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Marike Schiffer
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Winterton, A., Westlye, L.T., Steen, N.E. et al. Improving the precision of intranasal oxytocin research. Nat Hum Behav 5, 9–18 (2021). https://doi.org/10.1038/s41562-020-00996-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-020-00996-4
This article is cited by
-
An evolutionary timeline of the oxytocin signaling pathway
Communications Biology (2024)
-
Detection, processing and reinforcement of social cues: regulation by the oxytocin system
Nature Reviews Neuroscience (2023)
-
Oxytocin-pathway polygenic scores for severe mental disorders and metabolic phenotypes in the UK Biobank
Translational Psychiatry (2021)
-
The need for a reliable oxytocin assay
Molecular Psychiatry (2021)