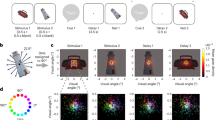
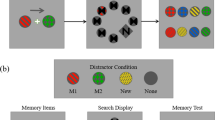
Abstract
Brain areas that control gaze are also recruited for covert shifts of spatial attention1,2,3,4,5,6,7,8,9. In the external space of perception, there is a natural ecological link between the control of gaze and spatial attention, as information sampled at covertly attended locations can inform where to look next2,10,11. Attention can also be directed internally to representations held within the spatial layout of visual working memory12,13,14,15,16. In such cases, the incentive for using attention to direct gaze disappears, as there are no external targets to scan. Here we investigate whether the oculomotor system of the brain also participates in attention focusing within the internal space of memory. Paradoxically, we reveal this participation through gaze behaviour itself. We demonstrate that selecting an item from visual working memory biases gaze in the direction of the memorized location of that item, despite there being nothing to look at and location memory never explicitly being probed. This retrospective ‘gaze bias’ occurs only when an item is not already in the internal focus of attention, and it predicts the performance benefit associated with the focusing of internal attention. We conclude that the oculomotor system also participates in focusing attention within memorized space, leaving traces all the way to the eyes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Code availability
Code is available from the authors on reasonable request.
Data availability
All data are publically available through the Dryad Digital Repository at: https://doi.org/10.5061/dryad.m99r286.
References
Rizzolatti, G., Riggio, L., Dascola, I. & Umiltá, C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia 25, 31–40 (1987).
Kustov, A. A. & Robinson, D. L. Shared neural control of attentional shifts and eye movements. Nature 384, 74–77 (1996).
Deubel, H. & Schneider, W. X. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis. Res. 36, 1827–1837 (1996).
Nobre, A. C. et al. Functional localization of the system for visuospatial attention using positron emission tomography. Brain 120, 515–533 (1997).
Moore, T. & Armstrong, K. M. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421, 370–373 (2003).
Moore, T., Armstrong, K. M. & Fallah, M. Visuomotor origins of covert spatial attention. Neuron 40, 671–683 (2003).
Muller, J. R., Philiastides, M. G. & Newsome, W. T. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc. Natl Acad. Sci. USA 102, 524–529 (2005).
Lovejoy, L. P. & Krauzlis, R. J. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat. Neurosci. 13, 261–266 (2010).
Krauzlis, R. J., Lovejoy, L. P. & Zénon, A. Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182 (2013).
Schall, J. D. & Hanes, D. P. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366, 467–469 (1993).
Zhou, H. & Desimone, R. Feature-based attention in the frontal eye field and area V4 during visual search. Neuron 70, 1205–1217 (2011).
Griffin, I. C. & Nobre, A. C. Orienting attention to locations in internal representations. J. Cogn. Neurosci. 15, 1176–1194 (2003).
Landman, R., Spekreijse, H. & Lamme, V. A. F. Large capacity storage of integrated objects before change blindness. Vis. Res. 43, 149–164 (2003).
Murray, A. M., Nobre, A. C., Clark, I. A., Cravo, A. M. & Stokes, M. G. Attention restores discrete items to visual short-term memory. Psychol. Sci. 24, 550–556 (2013).
Souza, A. S. & Oberauer, K. In search of the focus of attention in working memory: 13 years of the retro-cue effect. Atten. Percept. Psychophys. 78, 1839–1860 (2016).
van Ede, F., Niklaus, M. & Nobre, A. C. Temporal expectations guide dynamic prioritization in visual working memory through attenuated α oscillations. J. Neurosci. 37, 437–445 (2017).
Corneil, B. D. & Munoz, D. P. Overt responses during covert orienting. Neuron 82, 1230–1243 (2014).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Method 164, 177–190 (2007).
Müller, M. M., Teder-Sälejärvi, W. & Hillyard, S. A. The time course of cortical facilitation during cued shifts of spatial attention. Nat. Neurosci. 1, 631–634 (1998).
Busse, L., Katzner, S. & Treue, S. Temporal dynamics of neuronal modulation during exogenous and endogenous shifts of visual attention in macaque area MT. Proc. Natl Acad. Sci. USA 105, 16380–16385 (2008).
van Ede, F., de Lange, F. P. & Maris, E. Attentional cues affect accuracy and reaction time via different cognitive and neural processes. J. Neurosci. 32, 10408–10412 (2012).
Brandt, S. A. & Stark, L. W. Spontaneous eye movements during visual imagery reflect the content of the visual scene. J. Cogn. Neurosci. 9, 27–38 (1997).
Spivey, M. J. & Geng, J. J. Oculomotor mechanisms activated by imagery and memory: eye movements to absent objects. Psychol. Res. 65, 235–241 (2001).
Martarelli, C. S. & Mast, F. W. Eye movements during long-term pictorial recall. Psychol. Res. 77, 303–309 (2013).
Laeng, B., Bloem, I. M., D’Ascenzo, S. & Tommasi, L. Scrutinizing visual images: the role of gaze in mental imagery and memory. Cognition 131, 263–283 (2014).
Johansson, R. & Johansson, M. Look here, eye movements play a functional role in memory retrieval. Psychol. Sci. 25, 236–242 (2014).
Hafed, Z. M. & Clark, J. J. Microsaccades as an overt measure of covert attention shifts. Vis. Res. 42, 2533–2545 (2002).
Engbert, R. & Kliegl, R. Microsaccades uncover the orientation of covert attention. Vis. Res. 43, 1035–1045 (2003).
Lowet, E. et al. Enhanced neural processing by covert attention only during microsaccades directed toward the attended stimulus. Neuron 99, 207–214 (2018).
Hanning, N. M., Jonikaitis, D., Deubel, H. & Szinte, M. Oculomotor selection underlies feature retention in visual working memory. J. Neurophysiol. 115, 1071–1076 (2016).
Ohl, S. & Rolfs, M. Saccadic eye movements impose a natural bottleneck on visual short-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 43, 736–748 (2017).
Williams, M., Pouget, P., Boucher, L. & Woodman, G. F. Visual-spatial attention aids the maintenance of object representations in visual working memory. Mem. Cogn. 41, 698–715 (2013).
Ferreira, F., Apel, J. & Henderson, J. M. Taking a new look at looking at nothing. Trends Cogn. Sci. 12, 405–410 (2008).
Richardson, D. C. & Spivey, M. J. Representation, space and Hollywood Squares: looking at things that aren’t there anymore. Cognition 76, 269–295 (2000).
Martinez-Conde, S., Macknik, S. L. & Hubel, D. H. The role of fixational eye movements in visual perception. Nat. Rev. Neurosci. 5, 229–240 (2004).
Ahissar, E., Arieli, A., Fried, M. & Bonneh, Y. On the possible roles of microsaccades and drifts in visual perception. Vis. Res. 118, 25–30 (2016).
Awh, E. & Jonides, J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn. Sci. 5, 119–126 (2001).
Kuo, B.-C., Rao, A., Lepsien, J. & Nobre, A. C. Searching for targets within the spatial layout of visual short-term memory. J. Neurosci. 29, 8032–8038 (2009).
Dell’Acqua, R., Sessa, P., Toffanin, P., Luria, R. & Jolicœur, P. Orienting attention to objects in visual short-term memory. Neuropsychologia 48, 419–428 (2010).
Eimer, M. & Kiss, M. An electrophysiological measure of access to representations in visual working memory. Psychophysiology 47, 197–200 (2010).
Theeuwes, J., Kramer, A. F. & Irwin, D. E. Attention on our mind: the role of spatial attention in visual working memory. Acta Psychol. 137, 248–251 (2011).
Foster, J. J., Bsales, E. M., Jaffe, R. J. & Awh, E. Alpha-band activity reveals spontaneous representations of spatial position in visual working memory. Curr. Biol. 27, 3216–3223 (2017).
Schneegans, S. & Bays, P. M. Neural architecture for feature binding in visual working memory. J. Neurosci. 37, 3913–3925 (2017).
Umeno, M. M. & Goldberg, M. E. Spatial processing in the monkey frontal eye field. II. Memory responses. J. Neurophysiol. 86, 2344–2352 (2001).
Theeuwes, J., Belopolsky, A. & Olivers, C. N. L. Interactions between working memory, attention and eye movements. Acta Psychol. 132, 106–114 (2009).
Merrikhi, Y. et al. Spatial working memory alters the efficacy of input to visual cortex. Nat. Commun. 8, 15041 (2017).
van der Stigchel, S. & Hollingworth, A. Visuospatial working memory as a fundamental component of the eye movement system. Curr. Dir. Psychol. Sci. 27, 136–143 (2018).
van Ede, F., Chekroud, S. R., Stokes, M. G. & Nobre, A. C. Concurrent visual and motor selection during visual working memory guided action. Nat. Neurosci. https://doi.org/10.1038/s41593-018-0335-6 (2019).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J. M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Hentschke, H. & Stüttgen, M. C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 34, 1887–1894 (2011).
Acknowledgements
This research was funded by a Marie Skłodowska-Curie Fellowship from the European Commission (grant code: ACCESS2WM) to F.v.E., a Wellcome Trust Senior Investigator Award (grant number: 104571/Z/14/Z) and a James S. McDonnell Foundation Understanding Human Cognition Collaborative Award (grant number: 220020448) to A.C.N, and by the NIHR Oxford Health Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (grant number: 203139/Z/16/Z). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors also wish to thank A. Board and R. Silva Zunino for their help with the data collection of experiment 4.
Author information
Authors and Affiliations
Contributions
F.v.E. and A.C.N. conceived and designed the experiments. F.v.E. programmed the experiments. F.v.E. and S.R.C. acquired the data. F.v.E analysed the data. F.v.E., S.R.C. and A.C.N. interpreted the data. F.v.E. and A.C.N. drafted and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–4
Rights and permissions
About this article
Cite this article
van Ede, F., Chekroud, S.R. & Nobre, A.C. Human gaze tracks attentional focusing in memorized visual space. Nat Hum Behav 3, 462–470 (2019). https://doi.org/10.1038/s41562-019-0549-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-019-0549-y
This article is cited by
-
Pinging the brain to reveal the hidden attentional priority map using encephalography
Nature Communications (2023)
-
No obligatory trade-off between the use of space and time for working memory
Communications Psychology (2023)
-
Geometry of visuospatial working memory information in miniature gaze patterns
Nature Human Behaviour (2023)
-
Visual perceptual learning modulates microsaccade rate and directionality
Scientific Reports (2023)
-
A functional role for oculomotor preparation in mental arithmetic evidenced by the abducted eye paradigm
Psychological Research (2023)