Abstract
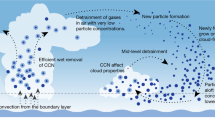
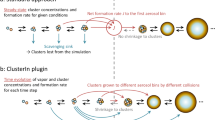
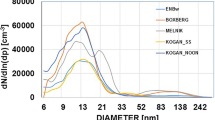
Aerosol particles in the atmosphere profoundly influence public health and climate. Ultrafine particles enter the body through the lungs and can translocate to essentially all organs, and they represent a major yet poorly understood health risk. Human activities have considerably increased aerosols and cloudiness since preindustrial times, but they remain persistently uncertain and underrepresented in global climate models. Here we present a synthesis of the current understanding of atmospheric new particle formation derived from laboratory measurements at the CERN CLOUD chamber. Whereas the importance of sulfuric acid has long been recognized, condensable vapours such as highly oxygenated organics and iodine oxoacids also play key roles, together with stabilizers such as ammonia, amines and ions from galactic cosmic rays. We discuss how insights from CLOUD experiments are helping to interpret new particle formation in different atmospheric environments, and to provide a mechanistic foundation for air quality and climate models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The CLOUD data are available from the published articles in the list of references and by request to their corresponding authors. Extended Data Table 2 provides the digital object identifier (doi) for selected CLOUD publications on nucleation and growth rates. The data for several CLOUD publications are already available on the Zenodo public repository (https://zenodo.org), hosted at CERN’s Data Centre, and the data for the remaining publications will shortly be made available on Zenodo.
References
Gordon, H. et al. Causes and importance of new particle formation in the present-day and preindustrial atmospheres. J. Geophys. Res. Atmos. 122, 8739–8760 (2017).
Kirkby, J. et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 476, 429–433 (2011).
Kürten, A., Williamson, C., Almeida, J., Kirkby, J. & Curtius, J. On the derivation of particle nucleation rates from experimental formation rates. Atmos. Chem. Phys. 15, 4063–4075 (2015).
Lee, S. et al. New particle formation in the atmosphere: from molecular clusters to global climate. J. Geophys. Res. Atmos. 124, 7098–7146 (2019).
Kerminen, V.-M. et al. Atmospheric new particle formation and growth: review of field observations. Environ. Res. Lett. 13, 103003 (2018).
Sellegri, K. et al. New particle formation: a review of ground-based observations at mountain research stations. Atmosphere 10, 493 (2019).
Zhang, R. et al. Formation of urban fine particulate matter. Chem. Rev. 115, 3803–3855 (2015).
O’Dowd, C. D. et al. Marine aerosol formation from biogenic iodine emissions. Nature 417, 632–636 (2002).
Yu, F. & Turco, R. P. From molecular clusters to nanoparticles: role of ambient ionization in tropospheric aerosol formation. J. Geophys. Res. 106, 4797–4814 (2001).
Schobesberger, S. et al. On the composition of ammonia–sulfuric-acid ion clusters during aerosol particle formation. Atmos. Chem. Phys. 15, 55–78 (2015).
Olenius, T., Kupiainen-Määttä, O., Ortega, I. K., Kurtén, T. & Vehkamäki, H. Free energy barrier in the growth of sulfuric acid–ammonia and sulfuric acid–dimethylamine clusters. J. Chem. Phys. 139, 084312 (2013).
Kürten, A. et al. Thermodynamics of the formation of sulfuric acid dimers in the binary (H2SO4−H2O) and ternary (H2SO4−H2O−NH3) system. Atmos. Chem. Phys. 15, 10701–10721 (2015).
Kürten, A. et al. Experimental particle formation rates spanning tropospheric sulfuric acid and ammonia abundances, ion production rates and temperatures. J. Geophys. Res. Atmos. 121, 12377–12400 (2016).
Bianchi, F. et al. New particle formation in the free troposphere: a question of chemistry and timing. Science 352, 1109–1112 (2016).
Bianchi, F. et al. The SALTENA experiment: comprehensive observations of aerosol sources, formation, and processes in the South American Andes. Bull. Am. Met. Soc. 103, E212–E229 (2022).
Yan, C. et al. The role of H2SO4−NH3 anion clusters in ion-induced aerosol nucleation mechanisms in the boreal forest. Atmos. Chem. Phys. 18, 13231–13243 (2018).
Jokinen, T. et al. Ion-induced sulfuric acid–ammonia nucleation drives particle formation in coastal Antarctica. Sci. Adv. 4, aat9744 (2018).
Lee, H. et al. Atmospheric new particle formation characteristics in the Arctic as measured at Mount Zeppelin, Svalbard, from 2016 to 2018. Atmos. Chem. Phys. 20, 13425–13441 (2020).
Sarnela, N. et al. Measurement-model comparison of stabilized Criegee intermediate and highly oxygenated molecule production in the CLOUD chamber. Atmos. Chem. Phys. 18, 2363–2380 (2018).
Shen, J. et al. High methanesulfonic acid production in the OH-initiated oxidation of dimethyl sulfide at low temperatures. Environ. Sci. Technol. https://doi.org/10.1021/acs.est.2c05154 (2022).
Chen, H. & Finlayson-Pitts, B. J. New particle formation from methanesulfonic acid and amines/ammonia as a function of temperature. Environ. Sci. Technol. 51, 243–252 (2016).
Almeida, J. et al. Molecular understanding of sulphuric acid−amine particle nucleation in the atmosphere. Nature 502, 359–363 (2013).
Kurtén, T., Loukonen, V., Vehkamäki, H. & Kulmala, M. Amines are likely to enhance neutral and ion-induced sulfuric acid−water nucleation in the atmosphere more effectively than ammonia. Atmos. Chem. Phys. 8, 4095–4103 (2008).
Kürten, A. et al. Neutral molecular cluster formation of sulfuric acid−dimethylamine observed in real time under atmospheric conditions. Proc. Natl Acad. Sci. USA 111, 15019–15024 (2014).
Cai, R. et al. The missing base molecules in atmospheric acid–base nucleation. Natl. Sci. Rev. 9, nwac137 (2022).
Bianchi, F. et al. Insight into acid–base nucleation experiments by comparison of the chemical composition of positive, negative, and neutral clusters. Environ. Sci. Technol. 48, 13675–13684 (2014).
Kürten, A. et al. New particle formation in the sulfuric acid−dimethylamine−water system: reevaluation of CLOUD chamber measurements and comparison to an aerosol nucleation and growth model. Atmos. Chem. Phys. 18, 845–863 (2018).
Stolzenburg, D. et al. Enhanced growth rate of atmospheric particles from sulfuric acid. Atmos. Chem. Phys. 20, 7359–7372 (2020).
Lehtipalo, K. et al. The effect of acid–base clustering and ions on the growth of atmospheric nano-particles. Nature Commun. 7, 11594 (2016).
Rondo, L. et al. Effect of dimethylamine on the gas phase sulfuric acid concentration measured by Chemical Ionization Mass Spectrometry (CIMS). J. Geophys. Res. Atmos. 121, 3036–3049 (2016).
Ge, X., Wexler, A. S. & Clegg, S. L. Atmospheric amines - Part I. A review. Atmos. Environ. 45, 524–546 (2011).
Hanson, D. R., McMurry, P. H., Jiang, J., Tanner, D. & Huey, L. G. Ambient pressure proton transfer mass spectrometry: detection of amines and ammonia. Environ. Sci. Technol. 45, 8881–8888 (2011).
Yao, L. et al. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science 361, 278–281 (2018).
Yin, R. et al. Acid–base clusters during atmospheric new particle formation in urban Beijing. Environ. Sci. Technol. 55, 10994–11005 (2021).
Smith, J. N. et al. Observations of aminium salts in atmospheric nanoparticles and possible climatic implications. Proc. Natl Acad. Sci. USA 107, 6634–6639 (2010).
Creamean, J. M. et al. Measurements of aerosol chemistry during new particle formation events at a remote rural mountain site. Environ. Sci. Technol. 45, 8208–8216 (2011).
Brean, J. et al. Open ocean and coastal new particle formation from sulfuric acid and amines around the Antarctic Peninsula. Nat. Geosci. 14, 383–388 (2021).
Sindelarova, K. et al. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 14, 9317–9341 (2014).
Molteni, U. et al. Formation of highly oxygenated organic molecules from α-pinene ozonolysis: chemical characteristics, mechanism, and kinetic model. ACS Earth Space Chem. 3, 873–883 (2019).
Bianchi, F. et al. Highly oxygenated organic molecules (HOM) from gas-phase autoxidation involving peroxy radicals: a key contributor to atmospheric aerosol. Chem. Rev. 119, 3472–3509 (2019).
Kirkby, J. et al. Ion-induced nucleation of pure biogenic particles. Nature 533, 521–526 (2016).
Zhang, R. et al. Atmospheric new particle formation enhanced by organic acids. Science 304, 1487–1490 (2004).
Elm, J., Myllys, N. & Kurtén, T. What is required for highly oxidized molecules to form clusters with sulfuric acid? J. Phys. Chem. A 121, 4578–4587 (2017).
Lehtipalo, K. et al. Multi-component new particle formation from sulfuric acid, ammonia, and biogenic vapors. Sci. Adv. 4, 12 (2018).
Frege, C. et al. Influence of temperature on the molecular composition of ions and charged clusters during pure biogenic nucleation. Atmos. Chem. Phys. 18, 65–79 (2018).
Simon, M. et al. Molecular understanding of new particle formation from α-pinene between −50 °C and 25 °C. Atmos. Chem. Phys. 20, 9183–9207 (2020).
Ye, Q. et al. Molecular composition and volatility of nucleated particles from α-pinene oxidation between −50 °C and +25 °C. Environ. Sci. Technol. 53, 12537–12365 (2019).
Tröstl, J. et al. The role of low-volatility organic compounds to initial particle growth in the atmosphere. Nature 533, 527–531 (2016).
Stolzenburg, D. et al. Rapid growth of organic aerosol nanoparticles over a wide tropospheric temperature range. Proc. Natl Acad. Sci. USA 115, 9122–9127 (2018).
Kulmala, M. et al. Direct observations of atmospheric aerosol nucleation. Science 339, 943–946 (2013).
McFiggans, G. et al. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 565, 587–593 (2019).
Heinritzi, M. et al. Molecular understanding of the suppression of new particle formation by isoprene. Atmos. Chem. Phys. 20, 11809–11821 (2020).
Yan, C. et al. Size-dependent influence of NOx on the growth rates of organic aerosol particles. Sci. Adv. 6, eaay4945 (2020).
Nie, W. et al. NO at low concentration can enhance the formation of highly oxygenated biogenic molecules in the atmosphere. Nat. Commun. 14, 3347 (2023).
Dada, L. et al. Role of sesquiterpenes in biogenic new particle formation. Sci. Adv. 9, eadi5297 (2023).
Andreae, M. O. et al. Aerosol characteristics and particle production in the upper troposphere over the Amazon Basin. Atmos. Chem. Phys. 18, 921–961 (2018).
Williamson, C. J. et al. A large source of cloud condensation nuclei from new particle formation in the tropics. Nature 574, 399–403 (2019).
Zha, Q. et al. Oxidized organic molecules in the tropical free troposphere over Amazonia. Natl Sci. Rev. https://doi.org/10.1093/nsr/nwad138 (2023).
Wang, X., Gordon, H., Grosvenor, D. P., Andreae, M. O. & Carslaw, K. S. Contribution of regional aerosol nucleation to low-level CCN in an Amazonian deep convective environment: results from a regionally nested global model. Atmos. Chem. Phys. 23, 4431–4461 (2023).
Carslaw, K. S. et al. Large contribution of natural aerosols to uncertainty in indirect forcing. Nature 503, 67–71 (2013).
Gordon, H. et al. Reduced anthropogenic aerosol radiative forcing caused by biogenic new particle formation. Proc. Natl Acad. Sci. USA 113, 12053–12058 (2016).
Rose, C. et al. Observations of biogenic ion-induced cluster formation in the atmosphere. Sci. Adv. 4, eaar5218 (2018).
Bianchi, F. et al. Biogenic particles formed in the Himalaya as an important source of free tropospheric aerosols. Nat. Geosci. 14, 4–9 (2020).
Andreae, M. O., Andreae, T. W., Ditas, F. & Pöhlker, C. Frequent new particle formation at remote sites in the subboreal forest of North America. Atmos. Chem. Phys. 22, 2487–2505 (2022).
Suni, T. et al. Formation and characteristics of ions and charged aerosol particles in a native Australian eucalypt forest. Atmos. Chem. Phys. 8, 129–139 (2008).
Wang, M. et al. Photo-oxidation of aromatic hydrocarbons produces low-volatility organic compounds. Environ. Sci. Technol. 54, 7911–7921 (2020).
Xiao, M. et al. The driving factors of new particle formation and growth in the polluted boundary layer. Atmos. Chem. Phys. 21, 14275–14291 (2021).
Sipilä, M. et al. Molecular-scale evidence of aerosol particle formation via sequential addition of HIO3. Nature 537, 532–534 (2016).
He, X.-C. et al. Role of iodine oxoacids in atmospheric aerosol nucleation. Science 371, 589–595 (2021).
Zhang, R. et al. Critical role of iodous acid in neutral iodine oxoacid nucleation. Environ. Sci. Technol. 56, 14166–14177 (2022).
Finkenzeller, H. et al. The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source. Nat. Chem. 15, 129–135 (2023).
Cuevas, C. A. et al. Rapid increase in atmospheric iodine levels in the North Atlantic since the mid-20th century. Nat. Commun. 9, 1452–1457 (2018).
Grose, M. R., Cainey, J. M., McMinn, A. & Gibson, J. A. E. Coastal marine methyl iodide source and links to new particle formation at Cape Grim during February 2006. Environ. Chem. 4, 172–177 (2007).
Mahajan, A. S. et al. Concurrent observations of atomic iodine, molecular iodine and ultrafine particles in a coastal environment. Atmos. Chem. Phys. 11, 2545–2555 (2011).
Yu, H. et al. Iodine speciation and size distribution in ambient aerosols at a coastal new particle formation hotspot in China. Atmos. Chem. Phys. 19, 4025–4039 (2019).
Baccarini, A. et al. Frequent new particle formation over the high Arctic pack ice by enhanced iodine emissions. Nat. Commun. 11, 4924 (2020).
Glasoe, W. A. et al. Sulfuric acid nucleation: an experimental study of the effect of seven bases. J. Geophys. Res. Atmos. 120, 1933–1950 (2015).
Schobesberger, S. et al. Molecular understanding of atmospheric particle formation from sulfuric acid and large oxidized organic molecules. Proc. Natl Acad. Sci. USA 110, 17223–17228 (2013).
Riccobono, F. et al. Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles. Science 344, 717–721 (2014).
Zapadinsky, E., Passananti, M., Myllys, N., Kurtén, T. & Vehkamäki, H. Modeling on fragmentation of clusters inside a mass spectrometer. J. Phys. Chem. A 123, 611–624 (2019).
Höpfner, M. et al. Ammonium nitrate particles formed in upper troposphere from ground ammonia sources during Asian monsoons. Nat. Geosci. 12, 608–612 (2019).
Ge, C., Zhu, C., Francisco, J. S., Zeng, X. C. & Wang, J. A molecular perspective for global modeling of upper atmospheric NH3 from freezing clouds. Proc. Natl Acad. Sci. USA 115, 6147–6152 (2018).
Wang, M. et al. Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation. Nature 605, 483–489 (2022).
Wang, M. & Kong, W. et al. Rapid growth of new atmospheric particles by nitric acid and ammonia condensation. Nature 581, 184–189 (2020).
Marten, R. et al. Survival of newly formed particles in haze conditions. Environ. Sci. Atmos. 2, 491–499 (2022).
Kulmala, M. et al. Atmospheric gas-to-particle conversion: why NPF events are observed in megacities? Faraday Disc. 200, 271–288 (2017).
Olenius, T., Yli-Juuti, T., Elm, J., Kontkanen, J. & Riipinen, I. in Physical Chemistry of Gas–Liquid Interfaces (eds. Faust, J. A. & House, J. E.) 315–352 (Elsevier, 2018).
Lovejoy, E. R., Curtius, J. & Froyd, K. D. Atmospheric ion-induced nucleation of sulfuric acid and water. J. Geophys. Res. Atmos. https://doi.org/10.1029/2003JD004460 (2004).
Ehrhart, S. et al. Comparison of the SAWNUC model with CLOUD measurements of sulphuric acid–water nucleation. J. Geophys. Res. Atmos. 121, 12401–12414 (2016).
McGrath, M. J. et al. Atmospheric Cluster Dynamics Code: a flexible method for solution of the birth–death equations. Atmos. Chem. Phys. 12, 2345–2355 (2012).
Yli-Juuti, T. et al. Model for acid–base chemistry in nanoparticle growth (MABNAG). Atmos. Chem. Phys. 13, 12507–12524 (2013).
Ahlm, L. et al. Modeling the thermodynamics and kinetics of sulfuric acid–dimethylamine–water nanoparticle growth in the CLOUD chamber. Aerosol Sci. Technol. 50, 1017–1032 (2016).
Määttänen, A. et al. New parameterizations for neutral and ion-induced sulfuric acid-water particle formation in nucleation and kinetic regimes. J. Geophys. Res. Atmos. 123, 1269–1296 (2018).
Yu, F. et al. H2SO4–H2O–NH3 ternary ion-mediated nucleation (TIMN): kinetic-based model and comparison with CLOUD measurements. Atmos. Chem. Phys. 18, 17451–17474 (2018).
Kürten, A. New particle formation from sulfuric acid and ammonia: nucleation and growth model based on thermodynamics derived from CLOUD measurements for a wide range of conditions. Atmos. Chem. Phys. 19, 5033–5050 (2019).
Dunne, E. et al. Global atmospheric particle formation from CERN CLOUD measurements. Science 354, 1119–1124 (2016).
Lelieveld, J. et al. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl Acad. Sci. USA 116, 7192–7197 (2019).
McCoy, I. L. et al. Influences of recent particle formation on Southern Ocean aerosol variability and low cloud properties. J. Geophys. Res. Atmos. 126, e2020JD033529 (2021).
Novelli, A. et al. Characterisation of an inlet pre-injector laser-induced fluorescence instrument for the measurement of atmospheric hydroxyl radicals. Atmos. Meas. Tech. 7, 3413–3430 (2014).
Schervish, M. & Donahue, N. M. Peroxy radical chemistry and the volatility basis set. Atmos. Chem. Phys. 20, 1183–1199 (2020).
Acknowledgements
We thank the many scientists who performed the CLOUD research reported here. We thank the European Organization for Nuclear Research (CERN) for supporting CLOUD with important technical and financial resources and for providing a particle beam from the CERN Proton Synchrotron. We thank our research institutes, national funding agencies and the European Union for providing financial support for the CLOUD experiment. We gratefully acknowledge financial support from the following sources: the German Ministry of Science and Education (CLOUD-22, 01LK2201), ACCC Flagship programme of the Academy of Finland (grant nos. 337549 and 337550), the Academy of Finland (grant no. 336557), the Swiss National Science Foundation (SNF grant no. 200021_213071), the Faculty of Physics at the University of Vienna, the University of Tartu (grant no. PRG714), Horizon 2020 (EMME-CARE, 857712), Horizon Europe MSCA-DN (CLOUD-DOC, 101073026), the NASA ROSES programme (grant no. 80NSSC19K0949) and the United States National Science Foundation (NSF grant nos. NSF-AGS-2215527, NSF-AGS-1602086, NSF-AGS-213208, NSF-AGS-2215489, NSF-AGS-2027252 and NSF-AGS-2215522).
Author information
Authors and Affiliations
Contributions
J.K., A.A., U.B., K.S.C, T.C., J.C, N.M.D., I.E.H., R.C.F., H.G., A.H., H.H., H.J., M.K., A.K., A.L., K.L., J.L., O.M., I.R., F.S., A.T., A.V., R.V., P.M.W. and D.R.W. contributed to the CLOUD research reported in the manuscript. J.K. wrote the manuscript. J.K. prepared Figs. 1–3, and N.M.D. prepared Fig. 4 together with J.K. All authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Song Guo, Fangqun Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Xujia Jiang, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kirkby, J., Amorim, A., Baltensperger, U. et al. Atmospheric new particle formation from the CERN CLOUD experiment. Nat. Geosci. 16, 948–957 (2023). https://doi.org/10.1038/s41561-023-01305-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-023-01305-0