Abstract
Increasing soil organic carbon contents contributes to global climate change mitigation. However, new plant inputs can enhance the mineralization of native soil organic carbon by the positive priming effect, which may counterbalance the sequestration of new carbon. Here we use soils from a 20 year chronosequence of inverted pasture soils (reciprocal translocation of topsoil and subsoil to >1 m) to study the dynamics of soil organic carbon in topsoils and subsoils. We evaluated the root-induced priming effect by differentiating native soil organic carbon from 13C root-derived carbon in a 6 month incubation experiment. We found that the addition of fresh root-derived carbon caused positive priming of native soil organic carbon in new topsoils (109 ± 27% additional respiration compared with controls without roots) and subsoils (331 ± 84%) after inversion. This effect was temporary for new topsoils as they accumulated soil organic carbon and adapted to high carbon inputs within a few years, leading to no priming in the long term. In contrast, buried topsoils became more sensitive to root carbon inputs over time, demonstrating how the legacy of high carbon inputs mediates the magnitude of priming (50% to 390% after 20 years of inversion). Overall, carbon losses with priming never exceeded new root-derived carbon inputs. We conclude that priming is a temporary reaction to additional carbon, which attenuates when soils adapt to high carbon inputs within a few years to decades.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available via Zenodo at https://doi.org/10.5281/zenodo.7304562.
References
Batjes, N. H. Harmonized soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks. Geoderma 269, 61–68 (2016).
Jobbágy, B. E. G. & Jackson, R. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Belowgr. Process. Glob. Change 10, 423–436 (2000).
Paustian, K. et al. Climate-smart soils. Nature 532, 49–57 (2016).
Minasny, B. et al. Soil carbon 4 per mille. Geoderma 292, 59–86 (2017).
Bossio, D. A. et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 3, 391–398 (2020).
Amelung, W. et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 11, 5427 (2020).
Lützow, M. V. et al. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur. J. Soil Sci. 57, 426–445 (2006).
Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011).
Peixoto, L. et al. Deep-rooted perennial crops differ in capacity to stabilize C inputs in deep soil layers. Sci. Rep. 12, 5952 (2022).
Thorup-Kristensen, K. et al. Digging deeper for agricultural resources, the value of deep rooting. Trends Plant Sci. 25, 406–417 (2020).
Button, E. S. et al. Deep-C storage: biological, chemical and physical strategies to enhance carbon stocks in agricultural subsoils. Soil Biol. Biochem. 170, 108697 (2022).
Kuzyakov, Y., Friedel, J. K. & Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 14, 382–395 (2000).
Blagodatskaya, Е & Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils 45, 115–131 (2008).
Guttières, R. et al. Temperature and soil management effects on carbon fluxes and priming effect intensity. Soil Biol. Biochem. 153, 108103 (2021).
Chen, L. et al. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 10, 5112 (2019).
Lyu, M. et al. Simulated leaf litter addition causes opposite priming effects on natural forest and plantation soils. Biol. Fertil. Soils 54, 925–934 (2018).
Siles, J. A. et al. Priming effects in soils across Europe. Glob. Change Biol. 28, 2146–2157 (2022).
Luo, Z., Wang, E. & Sun, O. J. A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems. Soil Biol. Biochem. 101, 96–103 (2016).
Bastida, F. et al. Global ecological predictors of the soil priming effect. Nat. Commun. 10, 3481 (2019).
Gaudel, G. et al. Meta-analysis of the priming effect on native soil organic carbon in response to glucose amendment across soil depths. Plant Soil 479, 107–124 (2022).
Fontaine, S. et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450, 277–280 (2007).
Fontaine, S., Bardoux, G., Abbadie, L. & Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 7, 314–320 (2004).
Bernard, L. et al. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 36, 1355–1377 (2022).
Guenet, B. et al. Impact of priming on global soil carbon stocks. Glob. Change Biol. 24, 1873–1883 (2018).
Wordell-Dietrich, P., Don, A. & Helfrich, M. Controlling factors for the stability of subsoil carbon in a Dystric Cambisol. Geoderma 304, 40–48 (2017).
Hicks Pries, C. E. et al. Root litter decomposition slows with soil depth. Soil Biol. Biochem. 125, 103–114 (2018).
Kuzyakov, Y. Review: factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 15, 382–395 (2002).
Kuzyakov, Y. & Blagodatskaya, E. Microbial hotspots and hot moments in soil: concept & review. Soil Biol. Biochem. 83, 184–199 (2015).
Huo, C., Luo, Y. & Cheng, W. Rhizosphere priming effect: a meta-analysis. Soil Biol. Biochem. 111, 78–84 (2017).
Dijkstra, F. A., Zhu, B. & Cheng, W. Root effects on soil organic carbon: a double‐edged sword. N. Phytol. 230, 60–65 (2021).
Kuzyakov, Y. & Razavi, B. S. Rhizosphere size and shape: temporal dynamics and spatial stationarity. Soil Biol. Biochem. 135, 343–360 (2019).
Kleber, M. et al. Dynamic interactions at the mineral-organic matter interface. Nat. Rev. Earth Environ. 2, 402–421 (2021).
Sokol, N. W. et al. Life and death in the soil microbiome: how ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 20, 415–430 (2022).
Sokol, N. W. & Bradford, M. A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 12, 46–53 (2019).
Jilling, A., Keiluweit, M., Gutknecht, J. L. M. & Grandy, A. S. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 158, 108265 (2021).
Schiedung, M., Tregurtha, C. S., Beare, M. H., Thomas, S. M. & Don, A. Deep soil flipping increases carbon stocks of New Zealand grasslands. Glob. Change Biol. 25, 2296–2309 (2019).
Alcántara, V., Don, A., Vesterdal, L., Well, R. & Nieder, R. Stability of buried carbon in deep-ploughed forest and cropland soils - implications for carbon stocks. Sci. Rep. 7, 5511 (2017).
Pereira, R. C. et al. Evidence for soil carbon enhancement through deeper mouldboard ploughing at pasture renovation on a Typic Fragiaqualf. Soil Res. 56, 182–191 (2018).
Kirschbaum, M. U. F. et al. Sequestration of soil carbon by burying it deeper within the profile: A theoretical exploration of three possible mechanisms. Soil Biol. Biochem. 163, 108432 (2021).
Alcántara, V., Don, A., Well, R. & Nieder, R. Deep ploughing increases agricultural soil organic matter stocks. Glob. Change Biol. 22, 2939–2956 (2016).
Sanaullah, M. et al. How do microbial communities in top- and subsoil respond to root litter addition under field conditions? Soil Biol. Biochem. 103, 28–38 (2016).
Cookson, W. R., Beare, M. H. & Wilson, P. E. Effects of prior crop residue management on microbial properties and crop residue decomposition. Appl. Soil Ecol. 7, 179–188 (1998).
Don, A., Böhme, I. H., Dohrmann, A. B., Poeplau, C. & Tebbe, C. C. Microbial community composition affects soil organic carbon turnover in mineral soils. Biol. Fertil. Soils 53, 445–456 (2017).
Lehmann, J. et al. Persitence of soil organic carbon caused by functional complexity. Nat. Geosci. 13, 529–534 (2020).
Fontaine, S., Mariotti, A. & Abbadie, L. The priming effect of organic matter: a question of microbial competition? Soil Biol. Biochem. 35, 837–843 (2003).
Kögel-Knabner, I. et al. Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 171, 61–82 (2008).
Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. Nature 528, 60–68 (2015).
Gregorich, E. G., Beare, M. H., McKim, U. F. & Skjemstad, J. O. Chemical and biological characteristics of physically uncomplexed organic matter. Soil Sci. Soc. Am. J. 70, 975–985 (2006).
Villarino, S. H., Pinto, P., Jackson, R. B. & Piñeiro, G. Plant rhizodeposition: a key factor for soil organic matter formation in stable fractions. Sci. Adv. 7, eabd3176 (2021).
Poeplau, C., Don, A. & Schneider, F. Roots are key to increasing the mean residence time of organic carbon entering temperate agricultural soils. Glob. Change Biol. 27, 4921–4934 (2021).
Fossum, C. et al. Belowground allocation and dynamics of recently fixed plant carbon in a California annual grassland. Soil Biol. Biochem. 165, 108519 (2022).
Sanderman, J., Creamer, C., Baisden, W. T., Farrell, M. & Fallon, S. Greater soil carbon stocks and faster turnover rates with increasing agricultural productivity. Soil 3, 1–16 (2017).
Angst, G., Kögel-Knabner, I., Kirfel, K., Hertel, D. & Mueller, C. W. Spatial distribution and chemical composition of soil organic matter fractions in rhizosphere and non-rhizosphere soil under European beech (Fagus sylvatica L.). Geoderma 264, 179–187 (2016).
Shi, A. et al. Substrate spatial heterogeneity reduces soil microbial activity. Soil Biol. Biochem. 152, 108068 (2021).
Nunan, N., Leloup, J., Ruamps, L. S., Pouteau, V. & Chenu, C. Effects of habitat constraints on soil microbial community function. Sci. Rep. 7, 4280 (2017).
Inagaki, T. M. et al. Microscale spatial distribution and soil organic matter persistence in top and subsoil. Soil Biol. Biochem. 178, 108921 (2023).
Lawrence‐Smith, E. J. et al. Full inversion tillage during pasture renewal to increase soil carbon storage: New Zealand as a case study. Glob. Change Biol. 27, 1998–2010 (2021).
Thomas, S. M., Beare, M. H. & Rietveld, V. Changes in soil quality following humping/hollowing and flipping of pakihi soils on the West Coast, South Island New Zealand. In Proceedings of the New Zealand Grassland Association 265–270 (New Zealand Grassland Association, 2007).
Anderson, J. P. E. & Domsch, K. H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10, 215–221 (1978).
Heinemeyer, O., Insam, H., Kaiser, E. A. & Walenzik, G. Soil microbial biomass and respiration measurements: an automated technique based on infra-red gas analysis. Plant Soil 195, 191–195 (1989).
Kaiser, E. A., Mueller, T., Joergensen, R. G., Insam, H. & Heinemeyer, O. Evaluation of methods to estimate the soil microbial biomass and the relationship with soil texture and organic matter. Soil Biol. Biochem. 24, 675–683 (1992).
Studer, M. S. et al. The MICE facility–a new tool to study plant–soil C cycling with a holistic approach. Isot. Environ. Health Stud. 53, 286–297 (2017).
Wollum, A. G. & Gomez, J. E. A conductivity method for measuring microbially evolved carbon dioxide. Ecology 51, 155–156 (1970).
Zimmermann, M., Leifeld, J., Schmidt, M. W. I., Smith, P. & Fuhrer, J. Measured soil organic matter fractions can be related to pools in the RothC model. Eur. J. Soil Sci. 58, 658–667 (2007).
Poeplau, C. et al. Reproducibility of a soil organic carbon fractionation method to derive RothC carbon pools. Eur. J. Soil Sci. 64, 735–746 (2013).
Abiven, S. & Andreoli, R. Charcoal does not change the decomposition rate of mixed litters in a mineral cambisol: a controlled conditions study. Biol. Fertil. Soils 47, 111–114 (2011).
Coplen, T. B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 25, 2538–2560 (2011).
R Studio (R Core Team, 2021).
Hothorn, T. et al. Multcomp package: simultaneous inference in general parametric models. R package version 1.4-17 (2021).
Acknowledgements
This work was funded by the Swiss National Science Foundation under project no. 200021_178768 (M.S. and S.A.) and by the European Union’s Horizon 2020 research and innovation programme as part of the EJPSoil project under grant agreement no. 862695 (A.D.). Additional support was provided by the Sustainable Agroecosystem programme at the New Zealand Institute for Plant and Food Research, with funding from the Strategic Science Investment Funded provided by the New Zealand Ministry for Business, Innovation and Employment (M.H.B). We thank C. Tregurtha and F. Hegewald for assistance with the soil sampling and S.-L. Bellè for his support in the laboratory. We also thank the soil organic matter group at the Thünen Institute of Climate-Smart Agriculture and the Research group on Organic Matter in soil and sediments at the Ecole Normale Supérieure Paris (ROMENS) for discussions and comments on earlier versions of the manuscript.
Author information
Authors and Affiliations
Contributions
M.S. conceptualized and designed the experiment with A.D., M.H.B. and S.A. M.S. conducted the experiment and performed all laboratory analyses. M.S. conducted the data analysis and wrote the manuscript with contributions from all authors. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Xujia Jiang, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Soil inversion and input of labelled roots.
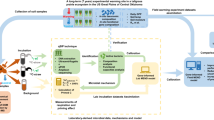
Concept of soil inversion (a) showing the non-inverted soil with SOC-rich topsoil. The inversion brings the former topsoil in the new subsoil and a new topsoil in created consisting of former subsoil. Set up of pre-incubation and growth of ryegrass under 13C enriched environment (10 atm%) to achieve highly labelled root input (b). The ryegrass seeds were placed on a 2 mm nylon mesh (black dotted line) to allow germination into the soil. After 22 days, aboveground biomass was removed directly at the mesh and soils were mixed prior to the division into the four replicates (equal weight) used for the closed jar incubation (c). The roots were cut during homogenization into 0.5-1 cm large pieces. The respired CO2 was trapped in NaOH traps to determine the total respiration by conductivity. By determining the isotopic composition of the trapped CO2 (precipitated as SrCO3), the contribution of native SOC and root-derived C was calculated (see method section). The isotopic signature of the rood-derived C allowed to trace it in the soils and within SOC fraction separated as particulate organic matter (POM) and mineral associated organic matter (MAOM; divided in sand & aggregate ( > 63 µm) and silt & clay ( < 63 µm) particle sizes).
Extended Data Fig. 2 Total CO2 production six months of incubation.
The cumulative total CO2-C production is shown per mass of soil (a & b) and the specific production is given per total SOC, including native SOC and root-derived C (c & d). All values are presented with the standard error of the mean of incubation replicates (n = 4, also shown as circles). Significant differences between the years since inversion were identified by analysis of variance (ANOVA) and p-values were computed on a 95% pairwise confidence interval with a post hoc-test (Tukey) and a Bonferroni multiplicity adjustment as indicated by letters (p < 0.05). All p-values are presented in Supplementary Table S6 and S7. The cumulative fluxes over the incubation time are shown in Supplementary Fig. S1.
Extended Data Fig. 3 Native SOC and root-derived C specific CO2 production.
Cumulative CO2 production over six months as native SOC specific [mg CO2-Cnative SOC (g native SOC)-1] respiration (a), root-derived C specific [mg CO2-Croot-derived SOC (g root-derived C)-1] (b) respiration and proportion of root-derived C (CO2-Croot-derived) of total respired CO2-C at each timestep (c) for topsoil and subsoils. Error bars indicate the propagated standard error of the mean over the days of incubation of the incubation replicates (n = 4).
Extended Data Fig. 4 Priming effect in inverted soils with roots.
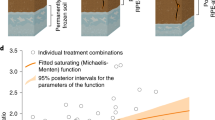
Additional native SOC respiration of inverted soils (3-20 years) with fitted two-tailed linear regression over time since inversion for topsoils (a; R² = 0.66, p < 0.001) and subsoils (b; R² = 0.14, p = 0.09). Grey linear model for subsoils (b; R² = 0.96, p < 0.001) shows the fit excluding the soil inverted seven years ago.
Extended Data Fig. 5 Incubated root-derived C in particulate organic matter.
Proportion of root-derived C remained after the incubation in the particulate organic matter (POM) fraction. The proportion of root derived C after the growth (pre-incubation) and before the incubation are shown in Supplementary Table S2. Linear models with time since inversion are shown in Supplementary Fig. S5.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schiedung, M., Don, A., Beare, M.H. et al. Soil carbon losses due to priming moderated by adaptation and legacy effects. Nat. Geosci. 16, 909–914 (2023). https://doi.org/10.1038/s41561-023-01275-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-023-01275-3