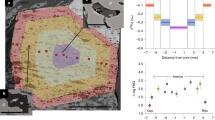
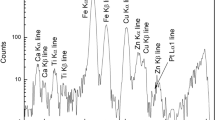
Abstract
Elevated H2O concentrations and oxygen fugacities are two fundamental properties that distinguish magmas formed in subduction zones from new crust generated at mid-ocean ridges. However, the mechanism of magma oxidation and how it relates to the increase in H2O remain unclear. In this study, we used infrared spectroscopy of mantle wedge orthopyroxene to trace the temporal and spatial evolution of oxygen fugacity during the transport of hydrous arc melts towards the crust. A positive correlation between equilibrium oxygen fugacity and orthopyroxene H2O concentrations for the peridotite samples studied allowed the assignment of specific, commonly observed absorption bands to redox-sensitive crystallographic defects. H2O content associated with these redox-sensitive defects increases in concentration across individual crystals, uniquely preserving the time-dependent transition from reduced to oxidized conditions during the migration of hydrous melts through the mantle wedge. A separate but related process of reaction with H2 occurring primarily during the earliest stages of melt–mantle reaction may be fundamental in generating the oxidized nature of hydrous melts. Our study proposes that the oxidized nature of arc magmas may not be a primary feature, but is instead acquired progressively as hydrous primary melts react with the surrounding mantle.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All supporting data for the manuscript are provided as a Supplementary Data file, and are also available online at https://doi.pangaea.de/10.1594/PANGAEA.902758. Any additional data may be requested from the authors.
References
Zimmer, M. M. et al. The role of water in generating the calc-alkaline trend: new volatile data for Aleutian magmas and a new tholeiitic index. J. Petrol. 51, 2411–2444 (2010).
Osborn, E. F. Role of oxygen pressure in the crystallization and differentiation of basaltic magma. Am. J. Sci. 257, 609–647 (1959).
Sisson, T. W. & Grove, T. L. Experimental investigations of the role of H2O in calc-alkaline differentiation and subduction zone magmatism. Contrib. Mineral. Petrol. 113, 143–166 (1993).
Campbell, I. H. & Taylor, S. R. No water, no granites—no oceans, no continents. Geophys. Res. Lett. 10, 1061–1064 (1983).
Kelley, K. A. & Cottrell, E. Water and the oxidation state of subduction zone magmas. Science 325, 605–607 (2009).
Pons, M.-L., Debret, B., Bouilhol, P., Delacour, A. & Williams, H. Zinc isotope evidence for sulfate-rich fluid transfer across subduction zones. Nat. Comm. 7, 13794 (2016).
Bénard, A. et al. Oxidising agents in sub-arc mantle melts link slab devolatilisation and arc magmas. Nat. Comm. 9, 3500 (2018).
Lee, C.-T. A., Leeman, W. P., Canil, D. & Li, Z.-X. A. Similar V/Sc systematics in MORB and arc basalts: implications for the oxygen fugacities of their mantle source regions. J. Petrol. 46, 2313–2336 (2005).
Lee, C.-T. A. et al. The redox state of arc mantle using Zn/Fe systematics. Nature 468, 681–685 (2010).
Mallmann, G. & St. C. O’Neill, H. The crystal/melt partitioning of V during mantle melting as a fuction of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). J. Petrol. 50, 1765–1794 (2009).
Burgisser, A. & Scaillet, B. Redox evolution of a degassing magma rising to the surface. Nature 445, 194–197 (2007).
Humphreys, M. C. S. et al. Coupled interactions between volatile activity and Fe oxidation state during arc crustal processes. J. Petrol. 56, 795–814 (2015).
Lee, C.-T. A. et al. Copper systematics in arc magmas and implications for crust–mantle differentiation. Science 336, 64–68 (2012).
Waters, L. E. & Lange, R. A. No effect of H2O degassing on the oxidation state of magmatic liquids. Earth Planet. Sci. Lett. 447, 48–59 (2016).
Tollan, P. M. E., St. C. O’Neill, H., Hermann, J., Benedictus, A. & Arculus, R. J. Frozen melt-rock reaction in a peridotite xenolith from sub-arc mantle recorded by diffusion of trace elements and water in olivine. Earth Planet. Sci. Lett. 422, 169–181 (2015).
Tollan, P. M. E., Dale, C. W., Hermann, J., Davidson, J. P. & Arculus, R. J. Generation and modification of the mantle wedge and lithosphere beneath the West Bismarck Island Arc: melting, metasomatism and thermal history of peridotite xenoliths from Ritter Island. J. Petrol. 58, 1475–1510 (2017).
Bénard, A., Woodland, A. B., Arculus, R. J., Nebel, O. & McAlpine, S. R. B. Variation in sub-arc mantle oxygen fugacity during partial melting recorded in refractory peridotite xenoliths from the West Bismarck Arc. Chem. Geol. 486, 16–30 (2018).
Wada, I. & Wang, K. Common depth of slab–mantle decoupling: reconciling diversity and uniformity of subduction zones. Geochem. Geophys. Geosyst. 10, Q10009 (2009).
Peslier, A. H., Luhr, J. F. & Post, J. Low water contents in pyroxenes from spinel-peridotites of the oxidized, sub-arc mantle wedge. Earth Planet. Sci. Lett. 201, 69–86 (2002).
Demouchy, S. & Bolfan-Casanova, N. Distribution and transport of hydrogen in the lithospheric mantle: a review. Lithos 240–243, 402–425 (2016).
Tian, Z.-Z. T. et al. Water concentration profiles in natural mantle orthopyroxenes: a geochronometer for long annealing of xenoliths within magma. Geology 45, 87–90 (2017).
Stalder, R. & Skogby, H. Dehydration mechanisms in synthetic Fe-bearing enstatite. Eur. J. Mineral. 19, 201–216 (2007).
Karner, J. M. et al. Valence state partitioning of Cr between pyroxene-melt: effects of pyroxene and melt compositions and direct determination of Cr valence states by XANES. Application to Martian basalt QUE 94201 composition. Am. Mineral. 92, 2002–2005 (2007).
Prechtel, F. & Stalder, R. OH-defects in Al- and Cr- doped synthetic enstatites and defect geobarometry on natural orthopyroxenes from Earth’s mantle. Eur. J. Mineral. 24, 471–481 (2012).
Demouchy, S. Diffusion of hydrogen in olivine grain boundaries and implications for the survival of water-rich zones in the Earth’s mantle. Earth Planet. Sci. Lett. 295, 305–313 (2010).
Brandon, A. D. & Draper, D. S. Constraints on the oxidation state of mantle overlying subduction zones: an example from Simcoe, Washington, USA. Geochim. Cosmochim. Acta 60, 1739–1749 (1996).
Frost, B. R. & Ballhaus, C. Comment on “Constraints on the origin of the oxidation state of mantle overlying subduction zones: an example from Simcoe, Washington, USA” by A.D. Brandon and D.S. Draper. Geochim. Cosmochim. Acta 62, 329–331 (1998).
Brandon, A. D. & Draper, D. S. Reply to the Comment by B.R. Frost and C. Ballhaus on “Constraints on the origin of the oxidation state of mantle overlying subduction zones: an example from Simcoe, Washington, USA”. Geochim. Cosmochim. Acta 62, 333–335 (1998).
Sambridge, M., Fitz Gerald, J., Kovács, I., St. C. O’Neill, H. & Hermann, J. Quantitative absorbance spectroscopy with unpolarized light: Part I. Physical and mathematical development. Am. Mineral. 93, 751–764 (2008).
Kovács, I. et al. Quantitative absorbance spectroscopy with unpolarized light: part II. Experimental evaluation and development of a protocol for quantitative analysis of mineral IR spectra. Am. Mineral. 93, 765–778 (2008).
Jackson, A., Parker, R. L., Sambridge, M., Constable, C. & Wolf, A. S. The inverse problem of unpolarized infrared spectroscopy of geological materials: estimation from noisy random sampling of a quadratic form. Am. Mineral. 103, 1176–1184 (2018).
Bell, D. R., Ihinger, P. D. & Rossman, G. R. Quantitative analysis of trace OH in garnet and pyroxenes. Am. Mineral. 80, 465–474 (1995).
Acknowledgements
We thank R. Arculus for providing the samples, and D. Rubatto and M. Jollands for discussions and feedback. This work was supported by the SNF project: 200021_169062.
Author information
Authors and Affiliations
Contributions
P.T. designed and conducted the FTIR measurements, and processed the data. P.T. and J.H. interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary information and Figs. 1–7
Supplementary Data
Supplementary data for orthopyroxene analyses.
Rights and permissions
About this article
Cite this article
Tollan, P., Hermann, J. Arc magmas oxidized by water dissociation and hydrogen incorporation in orthopyroxene. Nat. Geosci. 12, 667–671 (2019). https://doi.org/10.1038/s41561-019-0411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-019-0411-x
This article is cited by
-
Magmatic-tectonic response of the South China Craton to the Paleo-Pacific subduction during the Triassic: a new viewpoint based on Well NK-1
Journal of Oceanology and Limnology (2024)
-
Oxidation-induced nanolite crystallization triggered the 2021 eruption of Fukutoku-Oka-no-Ba, Japan
Scientific Reports (2023)
-
Slab-derived devolatilization fluids oxidized by subducted metasedimentary rocks
Nature Geoscience (2022)
-
Formation of oxidized sulfur-rich magmas in Neoarchaean subduction zones
Nature Geoscience (2022)
-
How fluid infiltrates dry crustal rocks during progressive eclogitization and shear zone formation: insights from H2O contents in nominally anhydrous minerals
Contributions to Mineralogy and Petrology (2022)