Abstract
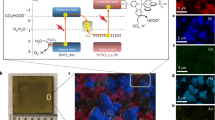
The synthesis of high-energy-density liquid fuels from CO2 and H2O powered by sunlight has the potential to create a circular economy. Despite the progress in producing simple gaseous products, the construction of unassisted photoelectrochemical devices for liquid multi-carbon production remains a major challenge. Here we assembled artificial leaf devices by integrating an oxide-derived Cu94Pd6 electrocatalyst with perovskite–BiVO4 tandem light absorbers that couple CO2 reduction with water oxidation. The wired Cu94Pd6|perovskite–BiVO4 tandem device provides a Faradaic efficiency of ~7.5% for multi-carbon alcohols (~1:1 ethanol and n-propanol), whereas the wireless standalone device produces ~1 µmol cm−2 alcohols after 20 h unassisted operation under air mass 1.5 G irradiation with a rate of ~40 µmol h−1 gCu94Pd6−1. This study demonstrates the direct production of multi-carbon liquid fuels from CO2 over an artificial leaf and, therefore, brings us a step closer to using sunlight to generate value-added complex products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw data supporting the findings of this study are available from the University of Cambridge data repository: https://doi.org/10.17863/CAM.95679.
References
Concepcion, J. J., House, R. L., Papanikolas, J. M. & Meyer, T. J. Chemical approaches to artificial photosynthesis. Proc. Natl Acad. Sci. USA 109, 15560–15564 (2012).
Hammarström, L. & Hammes-Schiffer, S. Artificial photosynthesis and solar fuels. Acc. Chem. Res. 42, 1859–1860 (2009).
Benson, E. E., Kubiak, C. P., Sathrum, A. J. & Smieja, J. M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 38, 89–99 (2009).
Zhuang, T.-T. et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Nat. Catal. 1, 946–951 (2018).
Rahaman, M., Dutta, A., Zanetti, A. & Broekmann, P. Electrochemical reduction of CO2 into multicarbon alcohols on activated Cu mesh catalysts: an identical location (IL) study. ACS Catal. 7, 7946–7956 (2017).
Zhuang, T.-T. et al. Steering post-C–C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi-carbon alcohols. Nat. Catal. 1, 421–428 (2018).
Ji, L. et al. Highly selective electrochemical reduction of CO2 to alcohols on an FeP nanoarray. Angew. Chem. Int. Ed. 59, 758–762 (2020).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Wang, Z. et al. Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2, 17135 (2017).
Swarnkar, A. et al. Quantum dot induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354, 92–95 (2016).
Wang, Z. et al. High irradiance performance of metal–halide perovskites for concentrator photovoltaics. Nat. Energy 3, 855–861 (2018).
Snaith, H. J. Present status and future prospects of perovskite photovoltaics. Nat. Mater. 17, 372–376 (2018).
Andrei, V. et al. Scalable triple-cation mixed-halide perovskite–BiVO4 tandems for bias-free water splitting. Adv. Energy Mater. 8, 1801403 (2018).
Zhang, H. et al. A sandwich-like organolead halide perovskite photocathode for efficient and durable photoelectrochemical hydrogen evolution in water. Adv. Energy Mater. 8, 1800795 (2018).
Pornrungroj, C. et al. Bifunctional perovskite–BiVO4 tandem devices for uninterrupted solar and electrocatalytic water splitting cycles. Adv. Funct. Mater. 31, 2008182 (2021).
Schreier, M. et al. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 6, 7326 (2015).
Gao, J. et al. Solar water splitting with perovskite/silicon tandem cell and TiC-supported Pt nanocluster electrocatalyst. Joule 3, 2930–2941 (2019).
Luo, J. et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 345, 1593–1596 (2014).
Zhang, H. et al. Carbon encapsulation of organic–inorganic hybrid perovskite toward efficient and stable photo-electrochemical carbon dioxide reduction. Adv. Energy Mater. 10, 2002105 (2020).
Bhattacharjee, S. et al. Reforming of soluble biomass- and plastic-derived waste using a bias-free Cu30Pd70|perovskite|Pt photoelectrochemical device. Adv. Funct. Mater. 32, 2109313 (2022).
Chen, J. et al. Compositionally screened eutectic catalytic coatings on halide perovskite photocathodes for photoassisted selective CO2 reduction. ACS Energy Lett. 4, 1279–1286 (2019).
Andrei, V., Reuillard, B. & Reisner, E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite–BiVO4 tandems. Nat. Mater. 19, 189–194 (2020).
Rahaman, M. et al. Selective CO production from aqueous CO2 using a Cu96In4 catalyst and its integration into a bias-free solar perovskite–BiVO4 tandem device. Energy Environ. Sci. 13, 3536–3543 (2020).
Huan, T. N. et al. Low-cost high-efficiency system for solar-driven conversion of CO2 to hydrocarbons. Proc. Natl Acad. Sci. USA 116, 9735–9740 (2019).
Gurudayal et al. Efficient solar-driven electrochemical CO2 reduction to hydrocarbons and oxygenates. Energy Environ. Sci. 10, 2222–2230 (2017).
Roh, I. et al. Photoelectrochemical CO2 reduction toward multicarbon products with silicon nanowire photocathodes interfaced with copper nanoparticles. J. Am. Chem. Soc. 144, 8002–8006 (2022).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Yu, J. et al. Recent progresses in electrochemical carbon dioxide reduction on copper-based catalysts toward multicarbon products. Adv. Funct. Mater. 31, 2102151 (2021).
Edwardes Moore, E. et al. A semi-artificial photoelectrochemical tandem leaf with a CO2-to-formate efficiency approaching 1%. Angew. Chem. Int. Ed. 60, 26303–26307 (2021).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Hori, Y., Wakebe, H., Tsukamoto, T. & Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 39, 1833–1839 (1994).
Dickinson, H. L. A. & Symes, M. D. Recent progress in CO2 reduction using bimetallic electrodes containing copper. Electrochem. Commun. 135, 107212 (2022).
Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Ma, S. et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu–Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 139, 47–50 (2017).
Lyu, Z. et al. Kinetically controlled synthesis of Pd–Cu janus nanocrystals with enriched surface structures and enhanced catalytic activities toward CO2 reduction. J. Am. Chem. Soc. 143, 149–162 (2021).
Rahaman, M. et al. Selective n-propanol formation from CO2 over degradation-resistant activated PdCu alloy foam electrocatalysts. Green Chem. 22, 6497–6509 (2020).
Li, J. et al. Enhanced multi-carbon alcohol electroproduction from CO via modulated hydrogen adsorption. Nat. Commun. 11, 3685 (2020).
Gao, D. F. et al. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J. Am. Chem. Soc. 137, 4288–4291 (2015).
Zheng, Y. et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 141, 7646–7659 (2019).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
van Santen, R. A., Neurock, M. & Shetty, S. G. Reactivity theory of transition-metal surfaces: a Brønsted−Evans−Polanyi linear activation energy−free-energy analysis. Chem. Rev. 110, 2005–2048 (2010).
Dutta, A. et al. CO2 electrolysis—complementary operando XRD, XAS and Raman spectroscopy study on the stability of CuxO foam catalysts. J. Catal. 389, 592–603 (2020).
Jiang, S., Klingan, K., Pasquini, C. & Dau, H. New aspects of operando Raman spectroscopy applied to electrochemical CO2 reduction on Cu foams. J. Chem. Phys. 150, 041718 (2019).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Toma, F. M. et al. Mechanistic insights into chemical and photochemical transformations of bismuth vanadate photoanodes. Nat. Commun. 7, 12012 (2016).
Uekert, T., Pichler, C. M., Schubert, T. & Reisner, E. Solar-driven reforming of solid waste for a sustainable future. Nat. Sustain. 4, 383–391 (2021).
Bhattacharjee, S. et al. Photoelectrochemical CO2-to-fuel conversion with simultaneous plastic reforming. Nat. Synth. 2, 182–192 (2023).
Nguyen, T. N. & Dinh, C.-T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 49, 7488–7504 (2020).
Allam, N. K. & Grimes, C. A. Electrochemical fabrication of complex copper oxide nanoarchitectures via copper anodization in aqueous and non-aqueous electrolytes. Mater. Lett. 65, 1949–1955 (2011).
Lu, H. et al. Single-source bismuth (transition metal) polyoxovanadate precursors for the scalable synthesis of doped BiVO4 photoanodes. Adv. Mater. 30, 1804033 (2018).
Acknowledgements
This work was supported by the European Commission with a Horizon 2020 Marie Skłodowska-Curie Individual European Fellowship (SolarFUEL, GAN 839763, M.R.), the Cambridge Trust (Vice-Chancellor’s Award to V.A., Cambridge Thai Foundation Award to C.P., HRH Prince of Wales Commonwealth Scholarship to S.B.), the Winton Programme for the physics of sustainability and St John’s College (Title A research fellowship to V.A.), the EPSRC (EP/L015978/1, EP/L027151/1, D.W., J.J.B., E.R.), the Swiss National Science Foundation (Early Postdoctoral Mobility fellowship P2EZP2-191791, E.L.) and the Austrian Science Fund (FWF, Schrödinger Fellowship J-4381, C.M.P). Access to the Cambridge XPS system, part of the Sir Henry Royce Institute-Cambridge equipment (EPSRC grant EP/P024947/1) and funding for TEM (EPSRC Underpinning Multi-User Equipment Call, EP/P030467/1) are gratefully acknowledged. We thank C. M. Fernández-Posada for assistance with the XPS and S. Kar and Y. Liu (University of Cambridge) for their valuable feedback on the paper.
Author information
Authors and Affiliations
Contributions
M.R. and E.R. designed the project. M.R. developed the bimetallic catalyst, assembled the artificial leaf devices and performed all the (photo)electrochemical experiments. V.A. prepared and characterized the perovskite and BiVO4 light absorbers and made the supplementary video. D.W. performed the operando Raman experiments. E.L. carried out the DFT calculations. C.P. assisted with oxygen analysis and schematic diagrams. S.B. and C.M.P. assisted with the catalyst characterization and analytics. H.F.G. helped with electron microscopy analyses. J.J.B provided insights in Raman measurements. M.R. and E.R. collectively wrote the paper with input from all co-authors. E.R. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Kazuhiro Takanabe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31, Tables 1–5 and References.
Supplementary Video 1
Evolution of gaseous products from photoanode and photocathode sides of a standalone artificial leaf under solar irradiation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahaman, M., Andrei, V., Wright, D. et al. Solar-driven liquid multi-carbon fuel production using a standalone perovskite–BiVO4 artificial leaf. Nat Energy 8, 629–638 (2023). https://doi.org/10.1038/s41560-023-01262-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01262-3
This article is cited by
-
Well-defined diatomic catalysis for photosynthesis of C2H4 from CO2
Nature Communications (2024)
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
Advancements on metal oxide semiconductor photocatalysts in photo-electrochemical conversion of carbon dioxide into fuels and other useful products
Frontiers in Energy (2024)