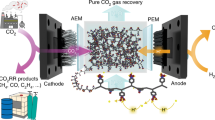
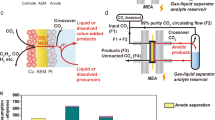
Abstract
CO2 electrolysis promises a route to carbon-based chemicals and fuels using renewable energy and resources. However, industrial application is limited by the transfer of CO2, electrons, protons and products (CEPP) at high current densities. Here we present an electrolyser that uses the forced convection of an aqueous CO2-saturated catholyte throughout a porous electrode and exploits the in situ formation of CO2(g)–liquid–catalyst interfaces to improve the CEPP transfer and reach high current densities. The CO2 supply is expedited by an increased exsolution of gaseous CO2 from dissolved CO2 and bicarbonate due to the effect of local pressure decreases; simultaneous CEPP transfer is promoted with a tenfold decrease in the diffusion layer thickness. This system also enables catalyst synthesis by in situ electrodeposition and ligand modification. We achieved a maximum current density of 3.37 A cm–2 with a Ag-based catalyst, and assemble a scaled-up 4 × 100 cm2 electrolyser stack that produces CO at a rate of 90.6 l h–1.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data are provided with this paper. Data generated and analysed in this study are included in the manuscript, Supplementary Information and Supplementary Data.
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Ross, M. B. et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2, 648–658 (2019).
Cano, Z. P. et al. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289 (2018).
Wen, G. et al. Ternary Sn–Ti–O electrocatalyst boosts the stability and energy efficiency of CO2 reduction. Angew. Chem. Int. Ed. 59, 12860–12867 (2020).
Wu, Y. et al. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 575, 639–642 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Koper, M. T. M. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 4, 2710 (2013).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Goyal, A. et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. J. Am. Chem. Soc. 142, 4154–4161 (2020).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Weekes, D. M. et al. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Shi, R. et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity. Nat. Catal. 4, 565–574 (2021).
Jiang, K. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018).
Ma, S. et al. Carbon nanotube containing Ag catalyst layers for efficient and selective reduction of carbon dioxide. J. Mater. Chem. A 4, 8573–8578 (2016).
Yang, H. et al. Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities. Nat. Commun. 11, 593 (2020).
Verma, S. et al. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 18, 7075–7084 (2016).
Garcia de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Endrodi, B. et al. Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolyzers. Nat. Energy 6, 439–448 (2021).
Ren, S. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 365, 367–369 (2019).
Grigioni, I. et al. CO2 electroreduction to formate at a partial current density of 930 mA cm–2 with InP colloidal quantum dot derived catalysts. ACS Energy Lett. 6, 79–84 (2020).
Fan, L. et al. Proton sponge promotion of electrochemical CO2 reduction to multi-carbon products. Joule 6, 205–220 (2022).
Li, Y. C. et al. CO2 electroreduction from carbonate electrolyte. ACS Energy Lett. 4, 1427–1431 (2019).
Gostick, J. T. et al. Pore network modeling of fibrous gas diffusion layers for polymer electrolyte membrane fuel cells. J. Power Sources 173, 277–290 (2007).
Zhao, X., Ren, H. & Luo, L. Gas bubbles in electrochemical gas evolution reactions. Langmuir 35, 5392–5408 (2019).
Liu, W. et al. A durable and pH-universal self-standing MoC–Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 12, 6776 (2021).
Clark, E. L. et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide. ACS Catal. 8, 6560–6570 (2018).
Wen, G. et al. Orbital interactions in Bi–Sn bimetallic electrocatalysts for highly selective electrochemical CO2 reduction toward formate production. Adv. Energy Mater. 8, 1802427 (2018).
Kok, M. D. R., Khalifa, A. & Gostick, J. T. Multiphysics simulation of the flow battery cathode: cell architecture and electrode optimization. J. Electrochem. Soc. 163, A1408–A1419 (2016).
Hashiba, H. et al. Effects of electrolyte buffer capacity on surface reactant species and the reaction rate of CO2 in electrochemical CO2 reduction. J. Phys. Chem. C. 122, 3719–3726 (2018).
Ringe, S. et al. Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on gold. Nat. Commun. 11, 33 (2020).
Singh, M. R. et al. Mechanistic insights into electrochemical reduction of CO2 over Ag using density functional theory and transport models. Proc. Natl Acad. Sci. USA 114, E8812–E8821 (2017).
Burdyny, T. et al. Nanomorphology-enhanced gas-evolution intensifies CO2 reduction electrochemistry. ACS Sustain. Chem. Eng. 5, 4031–4040 (2017).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2017).
Fang, Y. & Flake, J. C. Electrochemical reduction of CO2 at functionalized Au electrodes. J. Am. Chem. Soc. 139, 3399–3405 (2017).
Wang, Z. et al. Surface ligand promotion of carbon dioxide reduction through stabilizing chemisorbed reactive intermediates. J. Phys. Chem. Lett. 9, 3057–3061 (2018).
Nam, D. H. et al. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 19, 266–276 (2020).
Darling, R. M. & Perry, M. L. The influence of electrode and channel configurations on flow battery performance. J. Electrochem. Soc. 161, A1381–A1387 (2014).
Niu, Z.-Z. et al. Rigorous assessment of CO2 electroreduction products in a flow cell. Energy Environ. Sci. 14, 4169–4176 (2021).
Zheng, T. et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 3, 265–278 (2019).
Li, F. et al. Hierarchical mesoporous SnO2 nanosheets on carbon cloth: a robust and flexible electrocatalyst for CO2 reduction with high efficiency and selectivity. Angew. Chem. Int. Ed. 56, 505–509 (2017).
Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329–338 (2019).
Dunwell, M. et al. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold. J. Am. Chem. Soc. 139, 3774–3783 (2017).
Lin, C. et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012–1023 (2021).
Facq, S. et al. In situ Raman study and thermodynamic model of aqueous carbonate speciation in equilibrium with aragonite under subduction zone conditions. Geochim. Cosmochim. Acta 132, 375–390 (2014).
Negishi, N. et al. Effect of HCO3− concentration in groundwater on TiO2 photocatalytic water purification. Appl. Catal. B 242, 449–459 (2019).
Lu, Y. et al. Rechargeable K-CO2 Batteries with a KSn Anode and a Carboxyl-Containing Carbon Nanotube Cathode Catalyst. Angew. Chem. Int. Ed. 60, 9540–9545 (2021).
Chernyshova, I. V., Somasundaran, P. & Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA 115, E9261–E9270 (2018).
Weng, L. C., Bell, A. T. & Weber, A. Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 20, 16973–16984 (2018).
Wuttig, A. et al. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction. J. Am. Chem. Soc. 139, 17109–17113 (2017).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Sandberg, R. B. et al. CO–CO coupling on Cu facets: coverage, strain and field effects. Surf. Sci. 654, 56–62 (2016).
Wang, Y.-H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Lee, G. et al. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 6, 46–53 (2020).
Raciti, D., Livi, K. J. & Wang, C. Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett. 15, 6829–6835 (2015).
Li, G. et al. Design of ultralong single-crystal nanowire-based bifunctional electrodes for efficient oxygen and hydrogen evolution in a mild alkaline electrolyte. J. Mater. Chem. A 5, 10895–10901 (2017).
Wu, J. et al. Electrochemical reduction of carbon dioxide. J. Electrochem. Soc. 160, F953–F957 (2013).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Zhang, Z. et al. ‘Two ships in a bottle’ design for Zn–Ag–O catalyst enabling selective and long-lasting CO2 electroreduction. J. Am. Chem. Soc. 143, 6855–6864 (2021).
Ren, B. et al. Nano-crumples induced Sn-Bi bimetallic interface pattern with moderate electron bank for highly efficient CO2 electroreduction. Nat. Commun. 13, 2486 (2022).
Acknowledgements
We acknowledge the support from the University of Waterloo and the Natural Sciences and Engineering Research Council of Canada (NSERC). We gratefully thank the Canadian Light Source and its funding partners. We acknowledge the Vacuum Interconnected Nanotech Workstation (Nano-X) in the Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences (CAS). We acknowledge the financial support provided by the Outstanding Youth Project of the Guangdong Natural Science Foundation (grant no. 2021B1515020051, X.W.), Science and Technology Program of Guangzhou (2019050001, X.W.) and the National 111 Project. This work was also financially supported by the Department of Science and Technology of Guangdong Province (grant no. 2019JC01L203 and no. 2020B0909030004, X.W.) and the Natural Science Foundation of Guangdong Province (no. 2022A1515011804, G.W.). We acknowledge the computational resources from the Shared Hierarchical Academic Research Computing Network (SHARCNET, www.sharcnet.ca), Compute Canada and CMC Microsystems (http://www.cmc.ca).
Author information
Authors and Affiliations
Contributions
G.W. and Z.C. devised the idea and wrote the manuscript; X.W. and Z.C. supervised the project; G.W. and H.D. synthesized the materials; G.W. and R.G. performed the material and electrode characterizations; A.Y. guided and supervised the characterizations; G.W., B.R., D.L. and Y.Z. performed electrochemical experiments and data analyses; B.R. conducted the density functional theory simulations; G.W. and B.R. designed the cell structures; J.G. supervised the multiphysics simulations; all the authors engaged in the discussion and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–43, Notes 1–21, Methods, Tables 1–9 and references.
Supplementary Video 1
Startup of FTDT-cell for CO2RR.
Supplementary Video 2
FTDT-cell for CO2RR at 2.9 V with a total current of 25 A.
Supplementary Video 3
FTDT-cell for CO2RR at 3.5 V with a total current of 62 A.
Supplementary Video 4
FTDT-cell for CO2RR at 4.0 V with a total current of 90 A with two outlets.
Supplementary Data 1
Source Data for Supplementary Figures.
Source data
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 8
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, G., Ren, B., Wang, X. et al. Continuous CO2 electrolysis using a CO2 exsolution-induced flow cell. Nat Energy 7, 978–988 (2022). https://doi.org/10.1038/s41560-022-01130-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01130-6
This article is cited by
-
Visualization of CO2 electrolysis using optical coherence tomography
Nature Chemistry (2024)
-
Atomic Dispersed Hetero-Pairs for Enhanced Electrocatalytic CO2 Reduction
Nano-Micro Letters (2024)
-
High-rate and selective conversion of CO2 from aqueous solutions to hydrocarbons
Nature Communications (2023)
-
Hierarchical triphase diffusion photoelectrodes for photoelectrochemical gas/liquid flow conversion
Nature Communications (2023)