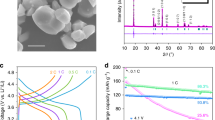
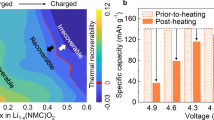
Abstract
Oxygen redox at high voltage has emerged as a transformative paradigm for high-energy battery cathodes such as layered transition-metal oxides by offering extra capacity beyond conventional transition-metal redox. However, these cathodes suffer from voltage hysteresis, voltage fade and capacity drop upon cycling. Single-crystalline cathodes have recently shown some improvements, but these challenges remain. Here we reveal the fundamental origin of oxygen redox instability to be from the domain boundaries that are present in single-crystalline cathode particles. By investigating single-crystalline cathodes with different domain boundaries structures, we show that the elimination of domain boundaries enhances the reversible lattice oxygen redox while inhibiting the irreversible oxygen release. This leads to significantly suppressed structural degradation and improved mechanical integrity during battery cycling and abuse heating. The robust oxygen redox enabled through domain boundary control provides practical opportunities towards high-energy, long-cycling, safe batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files.
References
Reed, J. & Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 104, 4513–4534 (2004).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Gent, W. E., Abate, I. I., Yang, W., Nazar, L. F. & Chueh, W. C. Design rules for high-valent redox in intercalation electrodes. Joule 4, 1369–1397 (2020).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2020).
Pearce, P. E. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 16, 580–586 (2017).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl. Acad. Sci. USA 112, 7650–7655 (2015).
Perez, A. J. et al. Approaching the limits of cationic and anionic electrochemical activity with the Li-rich layered rocksalt Li3IrO4. Nat. Energy 2, 954–962 (2017).
Maitra, U. et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 10, 288–295 (2018).
Rong, X. et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase transition. Joule 3, 503–517 (2019).
Wu, J. et al. Dissociate lattice oxygen redox reactions from capacity and voltage drops of battery electrodes. Sci. Adv. 6, eaaw3871 (2020).
Lee, E. & Persson, K. A. Structural and chemical evolution of the layered Li-excess LixMnO3 as a function of Li content from first-principles calculations. Adv. Energy Mater. 4, 1400498 (2014).
Kong, F. et al. Kinetic stability of bulk LiNiO2 and surface degradation by oxygen evolution in LiNiO2-based cathode materials. Adv. Energy Mater. 9, 1802586 (2019).
Yan, P. et al. Injection of oxygen vacancies in the bulk lattice of layered cathodes. Nat. Nanotechnol. 14, 602–608 (2019).
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Chen, Q. et al. Highly reversible oxygen redox in layered compounds enabled by surface polyanions. Nat. Commun. 11, 3411 (2020).
Zhang, X.-D. et al. Suppressing surface lattice oxygen release of Li-rich cathode materials via heterostructured spinel Li4Mn5O12 coating. Adv. Mater. 30, 1801751 (2018).
Fan, X. et al. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 70, 104450 (2020).
Li, J. et al. Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J. Electrochem. Soc. 164, A1534–A1544 (2017).
Qian, G. et al. Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 27, 140–149 (2020).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020).
Zhang, F. et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 11, 3050 (2020).
Xu, C. et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 20, 84–92 (2021).
Zhu, J. & Chen, G. Single-crystal based studies for correlating the properties and high-voltage performance of Li[NixMnyCo1−x−y]O2 cathodes. J. Mater. Chem. A 7, 5463–5474 (2019).
Klein, S. et al. Prospects and limitations of single-crystal cathode materials to overcome cross-talk phenomena in high-voltage lithium ion cells. J. Mater. Chem. A 9, 7546–7555 (2021).
Han, Y., Heng, S., Wang, Y., Qu, Q. & Zheng, H. Anchoring interfacial nickel cations on single-crystal LiNi0.8Co0.1Mn0.1O2 cathode surface via controllable electron transfer. ACS Energy Lett. 5, 2421–2433 (2020).
Pang, P. et al. Crack-free single-crystal LiNi0.83Co0.10Mn0.07O2 as cycling/thermal stable cathode materials for high-voltage lithium-ion batteries. Electrochim. Acta 365, 137380 (2021).
Fan, X. et al. Unravelling the influence of quasi single-crystalline architecture on high-voltage and thermal stability of LiNi0.5Co0.2Mn0.3O2 cathode for lithium-ion batteries. Chem. Eng. J. 393, 124709 (2020).
Li, N. et al. Unraveling the cationic and anionic redox reactions in a conventional layered oxide cathode. ACS Energy Lett. 4, 2836–2842 (2019).
Lee, G.-H. et al. Reversible anionic redox activities in conventional LiNi1/3Co1/3Mn1/3O2 cathodes. Angew. Chem. Int. Ed. 59, 8681–8688 (2020).
Lee, S.-Y. et al. Revisiting primary particles in layered lithium transition-metal oxides and their impact on structural degradation. Adv. Sci. 6, 1800843 (2019).
Jiang, Y. et al. Atomistic mechanism of cracking degradation at twin boundary of LiCoO2. Nano Energy 78, 105364 (2020).
Ahmed, S. et al. Understanding the formation of antiphase boundaries in layered oxide cathode materials and their evolution upon electrochemical cycling. Matter 4, 3953–3966 (2021).
Ge, M. et al. Kinetic limitations in single-crystal high-nickel cathodes. Angew. Chem. Int. Ed. 60, 17350–17355 (2021).
Geng, C. et al. Mechanism of action of the tungsten dopant in LiNiO2 positive electrode materials. Adv. Energy Mater. 12, 2103067 (2022).
Yang, W. & Devereaux, T. P. Anionic and cationic redox and interfaces in batteries: advances from soft X-ray absorption spectroscopy to resonant inelastic scattering. J. Power Sources 389, 188–197 (2018).
Dai, K. et al. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions. Joule 3, 518–541 (2019).
Wu, J. et al. Fingerprint oxygen redox reactions in batteries through high-efficiency mapping of resonant inelastic X-ray scattering. Condens. Matter 4, 5 (2019).
Zhuo, Z. et al. Spectroscopic signature of oxidized oxygen states in peroxides. J. Phys. Chem. Lett. 9, 6378–6384 (2018).
Yang, W. Oxygen release and oxygen redox. Nat. Energy 3, 619–620 (2018).
Liu, X. et al. Probing the thermal-driven structural and chemical degradation of Ni-rich layered cathodes by Co/Mn exchange. J. Am. Chem. Soc. 142, 19745–19753 (2020).
Rana, J. et al. Structural changes in Li2MnO3 cathode material for Li-ion batteries. Adv. Energy Mater. 4, 1300998 (2014).
Li, W., Asl, H. Y., Xie, Q. & Manthiram, A. Collapse of LiNi1−x−yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 141, 5097–5101 (2019).
Kondrakov, A. O. et al. Charge-transfer-induced lattice collapse in Ni-rich NCM cathode materials during delithiation. J. Phys. Chem. C. 121, 24381–24388 (2017).
Xu, C., Reeves, P. J., Jacquet, Q. & Grey, C. P. Phase behavior during electrochemical cycling of Ni-rich cathode materials for Li-ion batteries. Adv. Energy Mater. 11, 2003404 (2021).
Ren, D. et al. Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components. Appl. Energy 228, 633–644 (2018).
Yun, J. S. et al. Critical role of grain boundaries for ion migration in formamidinium and methylammonium lead halide perovskite solar cells. Adv. Energy Mater. 6, 1600330 (2016).
Polfus, J. M., Yildiz, B. & Tuller, H. L. Origin of fast oxide ion diffusion along grain boundaries in Sr-doped LaMnO3. Phys. Chem. Chem. Phys. 20, 19142–19150 (2018).
Qiao, R. et al. High-efficiency in situ resonant inelastic x-ray scattering (iRIXS) endstation at the Advanced Light Source. Rev. Sci. Instrum. 88, 033106 (2017).
Chuang, Y.-D. et al. Modular soft x-ray spectrometer for applications in energy sciences and quantum materials. Rev. Sci. Instrum. 88, 013110 (2017).
Hu, E. et al. Oxygen-redox reactions in LiCoO2 cathode without O–O bonding during charge-discharge. Joule 5, 720–736 (2021).
Deng, J. et al. The Velociprobe: an ultrafast hard X-ray nanoprobe for high-resolution ptychographic imaging. Rev. Sci. Instrum. 90, 083701 (2019).
Wakonig, K. et al. PtychoShelves, a versatile high-level framework for high-performance analysis of ptychographic data. J. Appl. Crystallogr. 53, 574–586 (2020).
Pelt, D. M. et al. Integration of TomoPy and the ASTRA toolbox for advanced processing and reconstruction of tomographic synchrotron data. J. Synchrotron Radiat. 23, 842–849 (2016).
E. Schwenker et al. Ingrained: an automated framework for fusing materials imaging simulations into experiments. GitHub https://github.com/MaterialEyes/ingrained-lite (2021).
He, X., Sun, H., Ding, X. & Zhao, K. Grain boundaries and their impact on Li kinetics in layered-oxide cathodes for Li-ion batteries. J. Phys. Chem. C. 125, 10284–10294 (2021).
Jain, A. et al. A high-throughput infrastructure for density functional theory calculations. Comput. Mater. Sci. 50, 2295–2310 (2011).
Jain, A. et al. Formation enthalpies by mixing GGA and GGA + U calculations. Phys. Rev. B 84, 045115 (2011).
Gale, J. D. GULP: a computer program for the symmetry-adapted simulation of solids. J. Chem. Soc. Faraday Trans. 93, 629–637 (1997).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA + U framework. Phys. Rev. B 73, 195107 (2006).
NIST Chemistry WebBook, NIST Standard Reference Database Number 69 (eds. Linstrom, P. J. & Mallard W. G.). NIST https://doi.org/10.18434/T4D303 (2021).
Acknowledgements
Research at the Argonne National Laboratory was funded by the US Department of Energy (DOE), Vehicle Technologies Office. Support from Tien Duong of the US DOE’s Office of Vehicle Technologies Program is gratefully acknowledged. Use of the Advanced Photon Source (APS) and the Centre for Nanoscale Materials, both Office of Science user facilities, was supported by the US Department of Energy, Office of Science and Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. Soft X-ray spectroscopy experiments were performed at the Advanced Light Source of the Lawrence Berkeley National Laboratory, a U.S. DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. X.L., G.-L.X., M.O. and K.A. thank the Clean Vehicle Consortium, US–China Clean Energy Research Centre (CERC-CVC2) for support. The authors thank G. Ceder for helpful discussion of DFT simulation on the OR behaviour of boundary-tailored layered cathodes, and B. Lai for technical support with ptychography characterization.
Author information
Authors and Affiliations
Contributions
G.-L.X. and X.L. conceived the idea and designed the experiments. G.-L.X., M.O. and K.A. initiated and supervised the project. X.L. synthesized and obtained all the battery materials. X.L. and C.Z. carried out the electrochemical tests. X.L., L.Y., A.D., Y.R. and Z.C. carried out in situ and ex situ XRD measurements and analysis. X.L. conducted the in situ heating HEXRD and mass spectroscopy with the assistance of W.X. Q.L., Z.Z. and W.Y. conducted the RIXS characterization and analysis on the samples prepared by X.L. and C.Z. W.L conducted synchrotron X-ray Laue diffraction characterization and analysis. X.Z., Y.L. and T.Z. performed the SEM and transmission electron microscopy characterization and analysis. D.R. and X.L. performed the ARC testing. X.L. and X.F. carried out the TGA-MS testing with different temperature rates. X.L., I.H. and C.S. performed the XAS measurement and analysis. J.D. performed X-ray ptychography characterization and analysis with the help of M.D. J.-J.F. conducted the DEMS under the supervision of L.H. and S.-G.S. V.S.C.K. and M.K.Y.C. performed DFT calculations. G.-L.X., X.L. and W.Y. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Jin Xie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figs. 1–30 and Tables 1–8.
Rights and permissions
About this article
Cite this article
Liu, X., Xu, GL., Kolluru, V.S.C. et al. Origin and regulation of oxygen redox instability in high-voltage battery cathodes. Nat Energy 7, 808–817 (2022). https://doi.org/10.1038/s41560-022-01036-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01036-3
This article is cited by
-
Structurally robust lithium-rich layered oxides for high-energy and long-lasting cathodes
Nature Communications (2024)
-
Suppressing strain propagation in ultrahigh-Ni cathodes during fast charging via epitaxial entropy-assisted coating
Nature Energy (2024)
-
In situ monitoring redox processes in energy storage using UV–Vis spectroscopy
Nature Energy (2023)
-
Defective oxygen inert phase stabilized high-voltage nickel-rich cathode for high-energy lithium-ion batteries
Nature Communications (2023)