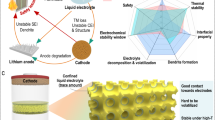
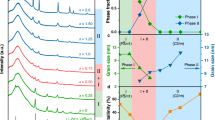
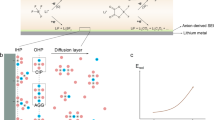
Abstract
To compete with commercial organic electrolytes, aqueous electrolytes beyond water-in-salt electrolytes with a lower salt concentration of <5.0 m (mol kgsolvent–1) and wider electrochemical stability window of >3.0 V are urgently needed. Here we report a 4.5 m lithium bis(trifluoromethanesulfonyl) imide (LiTFSI)–KOH–CO(NH2)2–H2O non-flammable ternary eutectic electrolyte that expands the electrochemical stability window to >3.3 V by forming a robust solid–electrolyte interphase. The ternary eutectic electrolyte enables Li1.5Mn2O4 || Li4Ti5O12 pouch cells to achieve a high average Coulombic efficiency of 99.96% and capacity retention of 92% after 470 cycles at an areal capacity of 2.5 mAh cm–2, a low positive/negative capacity ratio of 1.14 and a lean electrolyte (3 g Ah–1). The Li loss due to the solid–electrolyte interphase formation in the initial charge/discharge cycles is compensated by an excess 0.5 Li in the Li1.5Mn2O4 cathode, which converts the Li1.5Mn2O4 || Li4Ti5O12 cell into LiMn2O4 || Li4Ti5O12 after solid–electrolyte interphase formation. The 2.5 V aqueous Li1.5Mn2O4 || Li4Ti5O12 pouch cells with practical settings demonstrate a promising approach towards safe, low-cost and high-energy aqueous Li-ion batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files.
References
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Ueno, K. et al. Glyme–lithium salt equimolar molten mixtures: concentrated solutions or solvate ionic liquids? J. Phys. Chem. B 116, 11323–11331 (2012).
Wang, J. et al. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 7, 12032 (2016).
Borodin, O., Self, J., Persson, K. A., Wang, C. & Xu, K. Uncharted waters: super-concentrated electrolytes. Joule 4, 69–100 (2020).
Jiao, S. et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 3, 739–746 (2018).
Cao, Z., Hashinokuchi, M., Doi, T. & Inaba, M. Improved cycle performance of LiNi0.8Co0.1Mn0.1O2 positive electrode material in highly concentrated LiBF4/DMC. J. Electrochem. Soc. 166, A82–A88 (2019).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Yamada, Y. et al. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 1, 16129 (2016).
Suo, L. et al. Advanced high-voltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte. Angew. Chem. Int. Ed. 55, 7136–7141 (2016).
Wang, F. et al. Spinel LiNi0.5Mn1.5O4 cathode for high-energy aqueous lithium-ion batteries. Adv. Energy Mater. 7, 1600922 (2017).
Lukatskaya, M. R. et al. Concentrated mixed cation acetate “water-in-salt” solutions as green and low-cost high voltage electrolytes for aqueous batteries. Energy Environ. Sci. 11, 2876–2883 (2018).
Ko, S. et al. Lithium-salt monohydrate melt: a stable electrolyte for aqueous lithium-ion batteries. Electrochem. Commun. 104, 106488 (2019).
Chen, L. et al. A 63 m superconcentrated aqueous electrolyte for high-energy Li-ion batteries. ACS Energy Lett. 5, 968–974 (2020).
Droguet, L., Grimaud, A., Fontaine, O. & Tarascon, J. M. Water-in-salt electrolyte (WiSE) for aqueous batteries: a long way to practicality. Adv. Energy Mater. 10, 2002440 (2020).
Wang, F. et al. Hybrid aqueous/non-aqueous electrolyte for safe and high-energy Li-ion batteries. Joule 2, 927–937 (2018).
Chen, J. et al. Improving electrochemical stability and low-temperature performance with water/acetonitrile hybrid electrolytes. Adv. Energy Mater. 10, 1902654 (2020).
Yang, C. et al. 4.0 V aqueous Li-ion batteries. Joule 1, 122–132 (2017).
He, X. et al. Fluorine-free water-in-ionomer electrolytes for sustainable lithium-ion batteries. Nat. Commun. 9, 5320 (2018).
Xie, J., Liang, Z. & Lu, Y.-C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 19, 1006–1011 (2020).
Dubouis, N. et al. The role of the hydrogen evolution reaction in the solid–electrolyte interphase formation mechanism for “water-in-salt” electrolytes. Energy Environ. Sci. 11, 3491–3499 (2018).
Cordeiro, J. M. M. & Freitas, L. C. G. Study of water and dimethylformamide interaction by computer simulation. Z. Naturforsch. A 54, 110–116 (1999).
Qiu, H. et al. Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation. Nat. Commun. 10, 5374 (2019).
Zhao, J. et al. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 57, 625–634 (2019).
Hu, Z. et al. Nonflammable nitrile deep eutectic electrolyte enables high-voltage lithium metal batteries. Chem. Mater. 8, 3405–3413 (2020).
Rousseau, B., Alsenoy, C. V., Keuleers, R. & Desseyn, H. Solids modeled by ab-initio crystal field methods. Part 17. Study of the structure and vibrational spectrum of urea in the gas phase and in its P4̄21m crystal phase. J. Phys. Chem. A 102, 6540–6548 (1998).
Araujo, C. et al. Inelastic neutron scattering study of reline: shedding light on the hydrogen bonding network of deep eutectic solvents. Phys. Chem. Chem. Phys. 19, 17998–18009 (2017).
Keuleers, R., Desseyn, H., Rousseau, B. & Alsenoy, C. V. Vibrational analysis of urea. J. Phys. Chem. A 103, 4621–4630 (1999).
Finer, E., Franks, F. & Tait, M. Nuclear magnetic resonance studies of aqueous urea solutions. J. Am. Chem. Soc. 94, 4424–4429 (1972).
Sim, L., Yahya, R. & Arof, A. Infrared studies of polyacrylonitrile-based polymer electrolytes incorporated with lithium bis (trifluoromethane) sulfonimide and urea as deep eutectic solvent. Opt. Mater. 56, 140–144 (2016).
Zhu, B., Liang, Z. & Zou, R. Designing advanced catalysts for energy conversion based on urea oxidation reaction. Small 16, 1906133 (2020).
Xu, J. et al. Conductive carbon nitride for excellent energy storage. Adv. Mater. 29, 1701674 (2017).
Beamson, G. & Briggs, D. High Resolution XPS of Organic Polymers, the Scienta ESCA300 Database (Wiley, 1992).
Shaw, W. H. & Bordeaux, J. J. The decomposition of urea in aqueous media. J. Am. Chem. Soc. 77, 4729–4733 (1955).
Hou, Z. et al. Formation of solid–electrolyte interfaces in aqueous electrolytes by altering cation-solvation shell structure. Adv. Energy Mater. 10, 1903665 (2020).
Violante de Paz Ba´ñez, M., Aznar Moreno, J. A. & Galbis, J. A. Versatile sugar derivatives for the synthesis of potential degradable hydrophilic-hydrophobic polyurethanes and polyureas. J. Carbohydr. Chem. 27, 120–140 (2008).
Chen, J. et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386–397 (2020).
Smith, A., Burns, J. C., Zhao, X., Xiong, D. & Dahn, J. A high precision coulometry study of the SEI growth in Li/graphite cells. J. Electrochem. Soc. 158, A447–A452 (2011).
Diaz‐Lopez, M. et al. Li2O: Li–Mn–O disordered rock-salt nanocomposites as cathode prelithiation additives for high-energy density Li-ion batteries. Adv. Energy Mater. 10, 1902788 (2020).
Betz, J. et al. Theoretical versus practical energy: a plea for more transparency in the energy calculation of different rechargeable battery systems. Adv. Energy Mater. 9, 1803170 (2019).
Chao, D. et al. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci. Adv. 6, eaba4098 (2020).
Manalastas, W. Jr et al. Water in rechargeable multivalent-ion batteries: an electrochemical Pandora’s box. ChemSusChem 12, 379–396 (2019).
Acknowledgements
This work was financially supported by the US Department of Energy ARPA-E grant DEAR0000389.
Author information
Authors and Affiliations
Contributions
J.X. and C.W. conceived the idea for the project. J.X., J.Z. and C.Y. prepared the materials and performed the electrochemical experiments. X.J. conducted the quantum chemistry calculations and molecular dynamics simulations. J.X., P.W. and S.L. conducted the characterizations. K.L. and P.K. completed the differential scanning calorimetry tests. F.C. performed the NMR measurements. All the authors discussed the results, analysed the data and draughted the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.X., X.J. and C.H. are inventors on the US patent application (application number 2021-158-1) filed by the University of Maryland regarding the electrolytes described in this Article.
Peer review
Peer review information
Nature Energy thanks Maria Lukatskaya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–7, Tables 1–6 and Figs. 1–33.
Rights and permissions
About this article
Cite this article
Xu, J., Ji, X., Zhang, J. et al. Aqueous electrolyte design for super-stable 2.5 V LiMn2O4 || Li4Ti5O12 pouch cells. Nat Energy 7, 186–193 (2022). https://doi.org/10.1038/s41560-021-00977-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00977-5
This article is cited by
-
Water-in-polymer electrolyte with a wide electrochemical window and recyclability
Nature Sustainability (2024)
-
Trend of Developing Aqueous Liquid and Gel Electrolytes for Sustainable, Safe, and High-Performance Li-Ion Batteries
Nano-Micro Letters (2024)
-
Sulfolane-containing aqueous electrolyte solutions for producing efficient ampere-hour-level zinc metal battery pouch cells
Nature Communications (2023)
-
Water-in-salt electrolytes made saltier by Gemini ionic liquids for highly efficient Li-ion batteries
Scientific Reports (2023)
-
Designing electrolytes and interphases for high-energy lithium batteries
Nature Reviews Chemistry (2023)