Abstract
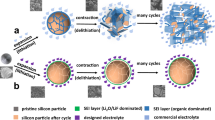
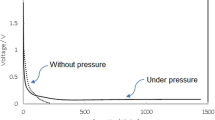
Due to the large volume variation of high-capacity alloy-based anodes during cycling, it is desirable to use small anode particles for an extended battery cycle life. However, it is still challenging to realize subnano-sized particles (<1 nm). Here we show a growth inhibition mechanism that prevents continuous enlargement of size immediately after nucleation during chemical vapour deposition. The growth inhibition is successfully applied to the synthesis of silicon, thereby yielding subnano-sized (<1 nm) silicon embedded in a highly stable dual matrix composed of carbon and silicon carbide. Ethylene not only functions as a silicon growth inhibitor, thereby slowing the growth of nucleated silicon via Si–C bond formation, but also acts as a source to create the dual matrix. The subnano-sized silicon anode enhances the cycling stability (Coulombic efficiency reaching 99.96% over 50 cycles). Finally, the practical application of the fabricated energy storage system (103.2 kWh) containing 110 Ah full-cells with 91% capacity retention for 2,875 cycles and a calendar life of 97.6% for 1 year is demonstrated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data generated and analysed in this study are included in the article and its Supplementary Information.
References
Chen, J. et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386–397 (2020).
Chae, S. et al. Gas phase synthesis of amorphous silicon nitride nanoparticles for high-energy LIBs. Energy Environ. Sci. 13, 1212–1221 (2020).
Lee, Y. et al. Stress relief principle of micron‐sized anodes with large volume variation for practical high‐energy lithium‐ion batteries. Adv. Funct. Mater. 30, 2004841 (2020).
Park, S.-H. et al. High areal capacity battery electrodes enabled by segregated nanotube networks. Nat. Energy 4, 560–567 (2019).
Son, Y. et al. Recent progress of analysis techniques for silicon-based anode of lithium-ion batteries. Curr. Opin. Electrochem. 6, 77–83 (2017).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 3, 31–35 (2008).
Shi, F. et al. Failure mechanisms of single-crystal silicon electrodes in lithium-ion batteries. Nat. Commun. 7, 11886 (2016).
Wu, H. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 7, 310–315 (2012).
Zhu, J. et al. Nanoporous silicon networks as anodes for lithium ion batteries. Phys. Chem. Chem. Phys. 15, 440–443 (2013).
Cho, J. et al. Negative electrode active material, method for producing same, and lithium secondary battery having negative electrode including same. PCT patent WO/2020/036397 (2020).
Cheng, Y.-T. et al. The influence of surface mechanics on diffusion induced stresses within spherical nanoparticles. J. Appl. Phys. 104, 083521 (2008).
Deshpande, R. et al. Modeling diffusion-induced stress in nanowire electrode structures. J. Power Sources 195, 5081–5088 (2010).
Jin, M. Y. et al. Optimum particle size in silicon electrodes dictated by chemomechanical deformation of the SEI. Adv. Funct. Mater. 31, 2010640 (2021).
Chen, S. et al. Scalable 2D mesoporous silicon nanosheets for high-performance lithium-ion battery anode. Small 14, 1703361 (2018).
Jiang, Y. et al. Hollow silica spheres with facile carbon modification as an anode material for lithium-ion batteries. J. Alloy. Compd. 744, 7–14 (2018).
Xie, J. et al. Core-shell yolk-shell Si@C@void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance. J. Power Sources 342, 529–536 (2017).
Kasukabe, T. et al. Beads-milling of waste Si sawdust into high-performance nanoflakes for lithium-ion batteries. Sci. Rep. 7, 42734 (2017).
Kim, H. et al. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 49, 2146–2149 (2010).
Ko, M. et al. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 1, 16113 (2016).
Liu, N. et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 9, 187–192 (2014).
Ma, D. D. D. et al. Small-diameter silicon nanowire surfaces. Science 299, 1874–1877 (2003).
McDowell, M. T. et al. Novel size and surface oxide effects in silicon nanowires as lithium battery anodes. Nano Lett. 11, 4018–4025 (2011).
Chae, S. et al. Integration of graphite and silicon anodes for the commercialization of high‐energy lithium‐ion batteries. Angew. Chem. Int. Ed. 59, 110–135 (2020).
Ma, J. et al. Towards maximized volumetric capacity via pore-coordinated design for large-volume-change lithium-ion battery anodes. Nat. Commun. 10, 475 (2019).
Ma, J. et al. Strategic pore architecture for accommodating volume change from high Si content in lithium‐ion battery anodes. Adv. Energy Mater. 10, 1903400 (2020).
Park, S. et al. Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat. Commun. 12, 838 (2021).
Park, S. et al. Scalable synthesis of hollow β-SiC/Si anodes via selective thermal oxidation for lithium-ion batteries. ACS Nano 14, 11548–11557 (2020).
Son, Y. et al. Calendering‐compatible macroporous architecture for silicon–graphite composite toward high‐energy lithium‐ion batteries. Adv. Mater. 32, 2003286 (2020).
Son, Y. et al. Quantification of pseudocapacitive contribution in nanocage-shaped silicon–carbon composite anode. Adv. Energy Mater. 9, 1803480 (2019).
Choi, S. et al. Scalable fracture-free SiOC glass coating for robust silicon nanoparticle anodes in lithium secondary batteries. Nano Lett. 14, 7120–7125 (2014).
Choi, S.-H. et al. Robust pitch on silicon nanolayer–embedded graphite for suppressing undesirable volume expansion. Adv. Energy Mater. 9, 1803121 (2018).
Li, Y. et al. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat. Energy 1, 15029 (2016).
Sung, J. et al. Fabrication of lamellar nanosphere structure for effective stress-management in large-volume-variation anodes of high-energy lithium-ion batteries. Adv. Mater. 31, 1900970 (2019).
Miyachi, M. et al. Analysis of SiO anodes for lithium-ion batteries. J. Electrochem. Soc. 152, A2089–A2091 (2005).
Suh, S.-S. et al. Electrochemical behavior of SiOx anodes with variation of oxygen ratio for Li-ion batteries. Electrochim. Acta 148, 111–117 (2014).
Prabhu, Y. T. et al. X-ray analysis by Williamson-Hall and size-strain plot methods of ZnO nanoparticles with fuel variation. World J. Nano Sci. Eng. 4, 21–28 (2014).
Rogers, K. D. et al. An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials 23, 2577–2585 (2002).
Obrovac, M. N. et al. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 114, 11444–11502 (2014).
Gaussian 16 v. A.03 (Gaussian, 2016).
Becke, A. D. et al. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993).
Lee, C., Yang, W. T. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Soria, F. A. et al. Si/C/H ReaxFF reactive potential for silicon surfaces grafted with organic molecules. J. Phys. Chem. C 122, 23515–23527 (2018).
Plimpton, S. et al. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Aktulga, H. M. et al. Parallel reactive molecular dynamics: numerical methods and algorithmic techniques. Parallel Comput. 38, 245–259 (2012).
Newsome, D. A. et al. Oxidation of silicon carbide by O2 and H2O: a ReaxFF reactive molecular dynamics study. Part 1. Phys. Chem. C 116, 16111–16121 (2012).
Šimonka, V. et al. ReaxFF reactive molecular dynamics study of orientation dependence of initial silicon carbide oxidation. J. Phys. Chem. A 121, 8791–8798 (2017).
Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning and the Ministry of Trade, Industry and Energy of the Republic of Korea (no. 20172410100140). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (2019R1A6A3A13095900). Fabrication of the 1 Ah and 110 Ah cells was supported by K. Kim and Samsung SDI.
Author information
Authors and Affiliations
Contributions
J.S., J.M. and N.K. conceived and designed the experiments. J.S., J.M. and N.K. prepared the samples and carried out the main experiments. J.H.L., S.H.J. and S.K.K. performed the simulation using MD and DFT. S.C. conducted dilatometry. M.Y. and J.H. participated in electrochemical measurements. T.L. and Y.L. assisted with sample preparation. J.S., N.K. and J.H.L. wrote the paper. J.C., N.K. and S.K.K. discussed the results and revised or commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have patents (J.C., J.S. and J.M., Patent Cooperation Treaty patent publication no. WO/2020/036397, published 20 February 2020. J.C., J.S. and J.M., Korean patent application no. 1021312620000, registered 1 July 2020. J.C., J.S. and J.M., Korean patent application no. 1022130820000, registered 1 February 2021. J.C. and J.S., Korean patent application no. 1021596930000, registered 18 September 2020.) related to the processes described in this article.
Additional information
Peer review information Nature Energy thanks Taylor Garrick and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–34, Notes 1–5, Tables 1–7 and references.
Rights and permissions
About this article
Cite this article
Sung, J., Kim, N., Ma, J. et al. Subnano-sized silicon anode via crystal growth inhibition mechanism and its application in a prototype battery pack. Nat Energy 6, 1164–1175 (2021). https://doi.org/10.1038/s41560-021-00945-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00945-z
This article is cited by
-
Standardized cycle life assessment of batteries using extremely lean electrolytic testing conditions
Communications Materials (2024)
-
Novel alginate-based binders for silicon–graphite anodes in lithium-ion batteries: effect of binder chemistry on the electrochemical performance
Journal of Applied Electrochemistry (2024)
-
Constructing a buffer macroporous architecture on silicon/carbon anode for high-performance lithium-ion battery
Journal of Materials Science: Materials in Electronics (2024)
-
Issues impeding the commercialization of laboratory innovations for energy-dense Si-containing lithium-ion batteries
Nature Energy (2023)
-
In Situ Formation of LiF-Rich Carbon Interphase on Silicon Particles for Cycle-Stable Battery Anodes
Transactions of Tianjin University (2023)