Abstract
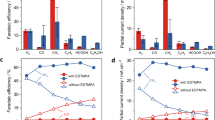
Electrochemical carbon dioxide reduction (CO2R) provides a promising pathway for sustainable generation of fuels and chemicals. Copper (Cu) electrocatalysts catalyse CO2R to valuable multicarbon (C2+) products, but their selectivity depends on the local microenvironment near the catalyst surface. Here we systematically explore and optimize this microenvironment using bilayer cation- and anion-conducting ionomer coatings to control the local pH (via Donnan exclusion) and CO2/H2O ratio (via ionomer properties), respectively. When this tailored microenvironment is coupled with pulsed electrolysis, further enhancements in the local ratio of CO2/H2O and pH are achieved, leading to selective C2+ production, which increases by 250% (with 90% Faradaic efficiency and only 4% H2) compared with static electrolysis over bare Cu. These results underscore the importance of tailoring the catalyst microenvironment as a means of improving overall performance in electrochemical syntheses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data for this study are available in the Supplementary Information. Source data are provided with this paper.
Change history
24 November 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41560-021-00960-0
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Verma, S. et al. Model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 9, 1972–1979 (2016).
Whipple, D. T. & Kenis, P. J. A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 1, 3451–3458 (2010).
Ebaid, M. et al. Production of C2/C3 oxygenates from planar copper nitride-derived mesoporous copper via electrochemical reduction of CO2. Chem. Mater. 32, 3304–3311 (2020).
Huang, Y., Chen, Y., Cheng, T., Wang, L.-W. & Goddard, W. A. Identification of the selective sites for electrochemical reduction of CO to C2+ products on copper nanoparticles by combining reactive force fields, density functional theory, and machine learning. ACS Energy Lett. 3, 2983–2988 (2018).
Jiang, K. et al. Effects of surface roughness on the electrochemical reduction of CO2 over Cu. ACS Energy Lett. 5, 1206–1214 (2020).
Bui, J. C., Kim, C., Weber, A. Z. & Bell, A. T. Dynamic boundary layer simulation of pulsed CO2 electrolysis on a copper catalyst. ACS Energy Lett. 6, 1181–1188 (2021).
Liu, X. et al. pH effects on the electrochemical reduction of CO(2) towards C2 products on stepped copper. Nat. Commun. 10, 32 (2019).
Wang, L. et al. Electrochemical carbon monoxide reduction on polycrystalline copper: effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal. 8, 7445–7454 (2018).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Clark, E. L. & Bell, A. T. in Carbon Dioxide Electrochemistry: Homogeneous and Heterogeneous Catalysis (eds Robert, M. et al.) Ch. 3 (Royal Society of Chemistry, 2020).
Resasco, J. & Bell, A. T. Electrocatalytic CO2 reduction to fuels: progress and opportunities. Trends Chem. 2, 825–836 (2020).
Singh, M. R., Clark, E. L. & Bell, A. T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide. Phys. Chem. Chem. Phys. 17, 18924–18936 (2015).
Kim, C., Weng, L.-C. & Bell, A. T. Impact of pulsed electrochemical reduction of CO2 on the formation of C2+ products over Cu. ACS Catal. 10, 12403–12413 (2020).
Kimura, K. W. et al. Selective electrochemical CO2 reduction during pulsed potential stems from dynamic interface. ACS Catal. 10, 8632–8639 (2020).
Wakerley, D. et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 18, 1222–1227 (2019).
Wei, X. et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces. ACS Catal. 10, 4103–4111 (2020).
Zhong, S. et al. Efficient electrochemical transformation of CO2 to C2/C3 chemicals on benzimidazole-functionalized copper surfaces. Chem. Commun. 54, 11324–11327 (2018).
Aeshala, L. M., Uppaluri, R. & Verma, A. Electrochemical conversion of CO2 to fuels: tuning of the reaction zone using suitable functional groups in a solid polymer electrolyte. Phys. Chem. Chem. Phys. 16, 17588–17594 (2014).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Gupta, K., Bersani, M. & Darr, J. A. Highly efficient electro-reduction of CO2 to formic acid by nano-copper. J. Mater. Chem. A 4, 13786–13794 (2016).
Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Sadeghpour, M., Yusoff, R. & Aroua, M. K. Polymeric ionic liquids (PILs) for CO2 capture. Rev. Chem. Eng. 33, 183–200 (2017).
Vermaas, D. A., Wiegman, S., Nagaki, T. & Smith, W. A. Ion transport mechanisms in bipolar membranes for (photo)electrochemical water splitting. Sustain. Energy Fuels 2, 2006–2015 (2018).
Lees, E. W. et al. Linking gas diffusion electrode composition to CO2 reduction in a flow cell. J. Mater. Chem. A 8, 19493–19501 (2020).
Wang, J. et al. Selective CO2 electrochemical reduction enabled by a tricomponent copolymer modifier on a copper surface. J. Am. Chem. Soc. 143, 2857–2865 (2021).
Xia, R. et al. Electrochemical reduction of acetonitrile to ethylamine. Nat. Commun. 12, 1949 (2021).
Pătru, A., Binninger, T., Pribyl, B. & Schmidt, T. J. Design principles of bipolar electrochemical co-electrolysis cells for efficient reduction of carbon dioxide from gas phase at low temperature. J. Electrochem. Soc. 166, F34–F43 (2019).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
S. H. Sichao Ma, et al. Electrolyzer and method of use. US patent 20200220185A1 (2020).
Blommaert, M. A. et al. Orientation of a bipolar membrane determines the dominant ion and carbonic species transport in membrane electrode assemblies for CO2 reduction. J. Mater. Chem. A 9, 11179–11186 (2021).
Mayerhöfer, B. et al. On the effect of anion exchange ionomer binders in bipolar electrode membrane interface water electrolysis. J. Mater. Chem. A 9, 14285–14295 (2021).
Bui, J. C., Digdaya, I., Xiang, C., Bell, A. T. & Weber, A. Z. Understanding multi-ion transport mechanisms in bipolar membranes. ACS Appl. Mater. Interfaces 12, 52509–52526 (2020).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Blanco, D. E., Lee, B. & Modestino, M. A. Optimizing organic electrosynthesis through controlled voltage dosing and artificial intelligence. Proc. Natl Acad. Sci. USA 116, 17683–17689 (2019).
Chong, X., Liu, C., Huang, Y., Huang, C. & Zhang, B. Potential-tuned selective electrosynthesis of azoxy-, azo- and amino-aromatics over a CoP nanosheet cathode. Natl Sci. Rev. 7, 285–295 (2019).
Barton, Z. J. et al. Electrochemical reduction selectivity of crotonaldehyde on copper. J. Appl. Electrochem. 51, 5–17 (2021).
Ren, Y. et al. Strategies to suppress hydrogen evolution for highly selective electrocatalytic nitrogen reduction: challenges and perspectives. Energy Environ. Sci. 14, 1176–1193 (2021).
Kusoglu, A., Dursch, T. J. & Weber, A. Z. Nanostructure/swelling relationships of bulk and thin-film PFSA ionomers. Adv. Funct. Mater. 26, 4961–4975 (2016).
Tesfaye, M., Kushner, D. I. & Kusoglu, A. Interplay between swelling kinetics and nanostructure in perfluorosulfonic acid thin-films: role of hygrothermal aging. ACS Appl. Polym. Mater. 1, 631–635 (2019).
Gierke, T. D., Munn, G. E. & Wilson, F. C. The morphology in Nafion perfluorinated membrane products, as determined by wide- and small-angle X-ray studies. J. Polym. Sci. Polym. Phys. Ed. 19, 1687–1704 (1981).
Shi, S., Weber, A. Z. & Kusoglu, A. Structure-transport relationship of perfluorosulfonic-acid membranes in different cationic forms. Electrochim. Acta 220, 517–528 (2016).
Zheng, Y. et al. Water uptake study of anion exchange membranes. Macromolecules 51, 3264–3278 (2018).
Ren, X., Myles, T. D., Grew, K. N. & Chiu, W. K. S. Carbon dioxide transport in Nafion 1100 EW membrane and in a direct methanol fuel cell. J. Electrochem. Soc. 162, F1221–F1230 (2015).
Zeebe, R. E. On the molecular diffusion coefficients of dissolved CO2,HCO3−, and CO32− and their dependence on isotopic mass. Geochim. Cosmochim. Acta 75, 2483–2498 (2011).
Crothers, A. R., Darling, R. M., Kusoglu, A., Radke, C. J. & Weber, A. Z. Theory of multicomponent phenomena in cation-exchange membranes: part II. Transport model and validation. J. Electrochem. Soc. 167, 013548 (2020).
Liu, Z., Yang, H., Kutz, R. & Masel, R. I. CO2. Electrolysis to CO and O2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 165, J3371–J3377 (2018).
Acknowledgements
This work was supported by the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the US Department of Energy under award no. DE-SC0004993 and Liquid Sunlight Alliance, which is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Fuels from Sunlight Hub under award no. DE-SC0021266.
Author information
Authors and Affiliations
Contributions
C.K. performed catalyst preparation, electrochemical experiments, characterizations and data interpretation. J.C.B. performed data interpretation and theoretical calculations. X.L. performed sample preparation and measurement for water uptake on ionomer film. J.K.C. performed X-ray photoemission spectroscopy analysis of ionomer-coated Cu. A.T.B., A.Z.W. and A.K. supervised the project. All authors discussed the results and participated in the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Feng Jiao and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17, Tables 1–3, Notes 1–6 and refs. 1–8.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Kim, C., Bui, J.C., Luo, X. et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat Energy 6, 1026–1034 (2021). https://doi.org/10.1038/s41560-021-00920-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00920-8
This article is cited by
-
Pulse potential mediated selectivity for the electrocatalytic oxidation of glycerol to glyceric acid
Nature Communications (2024)
-
Dynamics of bulk and surface oxide evolution in copper foams for electrochemical CO2 reduction
Communications Chemistry (2024)
-
Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction
Nature Communications (2024)
-
An organic approach
Nature Energy (2024)
-
Designing Membrane Electrode Assembly for Electrochemical CO2 Reduction: a Review
Transactions of Tianjin University (2024)