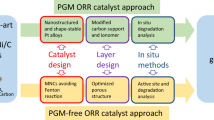
Abstract
Ultralow platinum loading and high catalytic performance at the membrane electrode assembly (MEA) level are essential for reducing the cost of proton exchange membrane fuel cells. The past decade has seen substantial progress in developing a variety of highly active platinum-based catalysts for the oxygen reduction reaction. However, these high activities are almost exclusively obtained from rotating disk electrode (RDE) measurements and have rarely translated into MEA performance. In this Review, we elucidate the intrinsic limitations that lead to a persistent failure to transfer catalysts’ high RDE activities into maximized MEA performance. We discuss catalyst-layer engineering strategies for controlling mass transport resistances at local catalyst sites, in the bulk of the catalyst layer and at the interfaces of the MEA to achieve high performance with ultralow platinum loading. We also examine promising intermediate testing methods for closing the gap between RDE and MEA experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
In the face of worsening climate crisis, UN Summit delivers new pathways and practical actions to shift global response into higher gear. United Nations https://www.un.org/sustainabledevelopment/blog/2019/2009/in-the-face-of-worsening-climate-crisis-un-summit-delivers-new-pathways-and-practical-actions-to-shift-global-response-into-higher-gear/ (2019).
Cano, Z. P. et al. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289 (2018).
Proton exchange membrane fuel cell (PEMFC) market to reach USD 47.60 billion by 2026; increasing government guidelines will boost growth. Unique Finance https://go.nature.com/3vFnIoz (2019).
Hydrogen Energy Ministerial Meeting 2019 (Ministry of Economy, Trade and Industry of Japan, 2019); https://img.fuelcellsworks.com/wp-content/uploads/2019/09/summary_en.pdf
Papageorgopoulos, D. Fuel Cell R&D Overview. 2019 Annual Merit Review and Peer Evaluation Meeting (Hydrogen and Fuel Cell Technologies Office, Office of Energy Efficiency and Renewable Energy, US Department of Energy, 2019); https://www.hydrogen.energy.gov/pdfs/review19/plenary_fuel_cell_papageorgopoulos_2019.pdf
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Banham, D., Choi, J. Y., Kishimoto, T. & Ye, S. Integrating PGM-free catalysts into catalyst layers and proton exchange membrane fuel cell devices. Adv. Mater. 31, 1804846 (2019).
Banham, D. et al. Critical advancements in achieving high power and stable nonprecious metal catalyst-based MEAs for real-word proton exchange membrane fuel cell applications. Sci. Adv. 4, 7180–7186 (2018).
DOE technical targets for polymer electrolyte membrane fuel cell components. US Department of Energy https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (2018).
Kong, F. et al. Active and stable Pt–Ni alloy octahedra catalyst for oxygen reduction via near-surface atomical engineering. ACS Catal. 10, 4205–4214 (2020).
Lim, J. et al. Ga-doped Pt–Ni octahedral nanoparticles as a highly active and durable electrocatalyst for oxygen reduction reaction. Nano Lett. 18, 2450–2458 (2018).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Gao, L. et al. Unconventional p–d hybridization interaction in PtGa ultrathin nanowires boosts oxygen reduction electrocatalysis. J. Am. Chem. Soc. 141, 18083–18090 (2019).
Alia, S. M. et al. Exceptional oxygen reduction reaction activity and durability of platinum-nickel nanowires through synthesis and post-treatment optimization. ACS Omega 2, 1408–1418 (2017).
Bu, L. et al. Biaxially strained PtPb Pt core shell nanoplate boosts oxygen reduction catalysis. Science 354, 1410–1414 (2016).
Tian, X. et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366, 850–856 (2019).
Zhang, L. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 349, 412–416 (2015).
Wang, X. et al. Pt-based icosahedral nanocages: using a combination of {111} facets, twin defects, and ultrathin walls to greatly enhance their activity toward oxygen reduction. Nano Lett. 16, 1467–1471 (2016).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Luo, S., Tang, M., Shen, P. K. & Ye, S. Atomic-scale preparation of octopod nanoframes with high-index facets as highly active and stable catalysts. Adv. Mater. https://doi.org/10.1002/adma.201601687 (2017).
Li, J. et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell. Catal. Joule 3, 124–135 (2019).
Zhang, Y. W. et al. High performance Pt monolayer catalysts produced via core-catalyzed coating in ethanol. ACS Catal. 4, 738–742 (2014).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 6, 241–247 (2007).
Wu, J. & Yang, H. Platinum-based oxygen reduction electrocatalyst. Acc. Chem. Res. 46, 1848–1857 (2013).
Cui, C. et al. Octahedral PtNi nanoparticle catalysts: exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 12, 5885–5889 (2012).
Choi, S. I. et al. Synthesis and characterization of 9 nm Pt-Ni octahedra with a record high activity of 3.3 A/mg(Pt) for the oxygen reduction reaction. Nano Lett. 13, 3420–3425 (2013).
Huang, X. et al. High-performance transition metal−doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Beermann, V. et al. Rh-doped Pt-Ni octahedral nanoparticles: understanding the correlation between elemental distribution, oxygen reduction reaction, and shape stability. Nano Lett. 16, 1719–1725 (2016).
Li, J. et al. Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 140, 2926–2932 (2018).
Wang, Q. et al. Sub-3 nm intermetallic ordered Pt3In clusters for oxygen reduction reaction. Adv. Sci. 7, 1901279–1901287 (2020).
Chong, L. et al. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Liang, J. et al. Biaxial strains mediated oxygen reduction electrocatalysis on fenton reaction resistant L10-PtZn fuel cell cathode. Adv. Energy Mater. 10, 2000179 (2020).
Inaba, M., Suzuki, T., Hantanaka, T. & Morimoto, Y. Fabrication and cell analysis of a Pt/SiO2 platinum thin film electrode. J. Electrochem. Soc. 162, F634–F638 (2015).
Gasteiger, H. A., Kocha, S. S., Sompalli, B. & Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 56, 9–35 (2005).
Schuler, T. et al. Fuel-cell catalyst-layer resistance via hydrogen limiting-current measurements. J. Electrochem. Soc. 166, F3020–F3031 (2019).
Kongkanand, A. & Mathias, M. F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 7, 1127–1137 (2016).
Ohma, A. et al. Analysis of proton exchange membrane fuel cell catalyst layers for reduction of platinum loading at Nissan. Electrochim. Acta 56, 10832–10841 (2011).
Pan, L., Ott, S., Dionigi, F. & Strasser, P. Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 18, 61–71 (2019).
Shi, S., Weber, A. Z. & Kusoglu, A. Structure–transport relationship of perfluorosulfonic-acid membranes in different cationic forms. Electrochim. Acta 220, 517–528 (2016).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Mohamed, H. F. M. et al. Free volume and permeabilities of O2 and H2 in Nafion membranes for polymer electrolyte fuel cells. Polymer 49, 3091–3097 (2008).
Myers, D., Kariuki, N., Ahluwalia, R., Wang, X. & Peng, J. K. Rationally Designed Catalyst Layers for PEMFC Performance Optimization (US DOE, 2015); https://www.hydrogen.energy.gov/pdfs/review15/fc106_myers_2015_o.pdf
Banas, C. J., Uddin, M. A., Park, J., Bonville, L. J. & Pasaogullari, U. Thinning of cathode catalyst layer in polymer electrolyte fuel cells due to foreign cation contamination. J. Electrochem. Soc. 165, F3015 (2018).
Debe, M. K. Tutorial on the fundamental characteristics and practical properties of nanostructured thin film (NSTF) catalysts. J. Electrochem. Soc. 160, F522–F534 (2013).
Kongkanand, A. et al. Development of dispersed-catalyst/NSTF hybrid electrode. J. Electrochem. Soc. 159, F676–F682 (2012).
Kongkanand, A. et al. Degradation of PEMFC observed on NSTF electrodes. J. Electrochem. Soc. 161, F744–F753 (2014).
Kodama, K. et al. Effect of the side-chain structure of perfluoro-sulfonic acid ionomers on the oxygen reduction reaction on the surface of Pt. ACS Catal. 8, 694–700 (2017).
Kudo, K., Jinnouchi, R. & Morimoto, Y. Humidity and temperature dependences of oxygen transport resistance of nafion thin film on platinum electrode. Electrochim. Acta 209, 682–690 (2016).
Ono, Y., Ohma, A., Shinohara, K. & Fushinobu, K. Influence of equivalent weight of ionomer on local oxygen transport. J. Electrochem. Soc. 160, F779–F787 (2013).
Rolfi, A., Oldani, C., Merlo, L., Facchhi, D. & Ruffo, R. New perfluorinated ionomer with improved oxygen permeability for application in cathode polymeric electrolyte membrane fuel cell. J. Power Sources 396, 95–101 (2018).
Snyder, J., Livi, K. & Erlebacher, J. Oxygen reduction reaction performance of [MTBD][beti]-encapsulated nanoporous NiPt alloy nanoparticles. Adv. Funct. Mater. 23, 5494–5501 (2013).
Zhao, Z. et al. Tailoring a three-phase microenvironment for high-performance oxygen reduction reaction in proton exchange membrane fuel cells. Matter 3, 1774–1790 (2020).
Ott, S. et al. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 19, 77–85 (2020).
Yang, C. et al. Nitrogen and sulfur co-doped porous carbon sheets for energy storage and pH-universal oxygen reduction reaction. Nano Energy 54, 192–199 (2018).
Xia, W. et al. Highly ordered macroporous dual-element-doped carbon from metal–organic frameworks for catalyzing oxygen reduction. Chem. Sci. 11, 9584–9592 (2020).
Chokradjaroen, C. et al. A comparative study of undoped, boron-doped, and boron/fluorine dual-doped carbon nanoparticles obtained via solution plasma as catalysts for the oxygen reduction reaction. Sustain. Energy Fuels 4, 4570–4580 (2020).
Chaisubanan, N. et al. Insight into the alternative metal oxide modified carbon-supported PtCo for oxygen reduction reaction in proton exchange membrane fuel cell. Renew. Energy 139, 679–687 (2019).
Yu, X. et al. Coupling of iron phthalocyanine at carbon defect site via π–π stacking for enhanced oxygen reduction reaction. Appl. Catal. B 280, 119437 (2021).
Goswami, C., Hazarika, K. K. & Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 1, 117–128 (2018).
Orfanidi, A. et al. The key to high performance low Pt loaded electrodes. J. Electrochem. Soc. 164, F418–F426 (2017).
Doo, G. et al. Nano-scale control of the ionomer distribution by molecular masking of the Pt surface in PEMFCs. J. Mater. Chem. A 8, 13004–13013 (2020).
Wang, J. X., Springer, T. E. & Adzic, R. R. Dual-pathway kinetic equation for the hydrogen oxidation reaction on Pt electrodes. J. Electrochem. Soc. 153, A1732 (2006).
Gasteiger, H. A., Panels, J. E. & Yan, S. G. Dependence of PEM fuel cell performance on catalyst loading. J. Power Sources 127, 162–171 (2004).
Banham, D. & Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: an industrial perspective. ACS Energy Lett. 2, 629–638 (2017).
Ramaswamy, N. & Kumaraguru, S. Materials and design selection to improve high current density. ECS Trans. 2018, 835–842 (2018).
Park, Y., Tokiwa, H., Kakinuma, K., Watanabe, M. & Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 315, 179–191 (2016).
Yarlagadda, V. et al. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 3, 618–621 (2018).
Banham, D. et al. Novel mesoporous carbon supports for PEMFC. Catalysts 5, 1046–1067 (2015).
Takeshita, T., Kamitaka, Y., Shinozaki, K., Kodama, K. & Morimoto, Y. Evaluation of ionomer coverage on Pt catalysts in polymer electrolyte membrane fuel cells by CO stripping voltammetry and its effect on oxygen reduction reaction activity. J. Electroanal. Chem. 871, 114250 (2020).
Welch, C. et al. Nafion in dilute solvent systems: dispersion or solution? ACS Macro Lett. 1, 1403–1407 (2012).
Xu, H. Ionomer Dispersion Impact on PEM Fuel Cell and Electrolyzer Performance and Durability 2019 DOE H2 and Fuel Cell Annual Merit Review Meeting (US DOE, 2019); https://www.hydrogen.energy.gov/pdfs/review19/fc117_xu_2019_p.pdf
Wang, M. et al. Impact of catalyst ink dispersing methodology on fuel cell performance using in-situ X-ray scattering. ACS Appl. Energ. Mater. 2, 6417–6427 (2019).
Li, Y. et al. Carbon corrosion behaviors and the mechanical properties of proton exchange membrane fuel cell cathode catalyst layer. Int. J. Hydrog. Energy 45, 23519–23525 (2020).
Sassin, M. B., Garsany, Y., Atkinson, R. W., Hjelm, R. M. E. & Swider-Lyons, K. E. Understanding the interplay between cathode catalyst layer porosity and thickness on transport limitations en route to high-performance PEMFCs. Int. J. Hydrogen Energy 44, 16944–16955 (2019).
Suzuki, A. et al. Ionomer content in the catalyst layer of polymer electrolyte membrane fuel cell (PEMFC): effects on diffusion and performance. Int. J. Hydrogen Energy 36, 2221–2229 (2011).
Yoshino, S., Shinohara, A., Kodama, K. & Morimoto, Y. Fabrication of catalyst layer with ionomer nanofiber scaffolding for polymer electrolyte fuel cells. J. Power Sources 476, 228584 (2020).
Slack, J. J. et al. Nanofiber fuel cell MEAs with a PtCo/C cathode. J. Electrochem. Soc. 166, F3202–F3209 (2019).
Rabat, H. & Brault, P. Plasma sputtering deposition of PEMFC porous carbon platinum electrodes. Fuel Cells 8, 81–86 (2008).
Ramaswamy, N. et al. Enhanced activity and interfacial durability study of ultra low Pt based electrocatalysts prepared by ion beam assisted deposition (IBAD) method. Electrochim. Acta 54, 6756–6766 (2009).
Shukla, S., Domican, K., Karan, K., Bhattacharjee, S. & Secanell, M. Analysis of low platinum loading thin polymer electrolyte fuel cell electrodes prepared by inkjet printing. Electrochim. Acta 156, 289–300 (2015).
Ercolano, G., Farina, F., Cavaliere, S., Jones, D. J. & Rozière, J. Towards ultrathin Pt films on nanofibres by surface-limited electrodeposition for electrocatalytic applications. J. Mater. Chem. A 5, 3974–3980 (2017).
Kayarkatte, M. K., Delikaya, Ö. & Roth, C. Freestanding catalyst layers: a novel electrode fabrication technique for PEM fuel cells via electrospinning. ChemElectroChem 4, 404–411 (2017).
Breitwieser, M., Klingele, M., Vierrath, S., Zengerle, R. & Thiele, S. Tailoring the membrane–electrode interface in PEM fuel cells: a review and perspective on novel engineering approaches. Adv. Energy Mater. 8, 1701257 (2018).
Koh, J. K., Jeon, Y., Cho, Y. I., Kim, J. H. & Shul, Y. A facile preparation method of surface patterned polymer electrolyte membranes for fuel cell applications. J. Mater. Chem. A 2, 8652–8659 (2014).
Joseph, D. et al. Porous nafion membranes. J. Membr. Sci. 520, 723–730 (2016).
Dang, Q. K. et al. Nafion membranes with a porous surface. J. Membr. Sci. 460, 199–205 (2014).
Klingele, M., Breitwieser, M., Zengerle, R. & Thiele, S. Direct deposition of proton exchange membranes enabling high performance hydrogen fuel cells. J. Mater. Chem. A 3, 11239–11245 (2015).
Klingele, M. et al. A completely spray-coated membrane electrode assembly. Electrochem. Commun. 70, 65–68 (2016).
Hizir, F. E., Ural, S. O., Kumbur, E. C. & Mench, M. M. Characterization of interfacial morphology in polymer electrolyte fuel cells: micro-porous layer and catalyst layer surfaces. J. Power Sources 195, 3463–3471 (2010).
Aoyama, Y., Suzuki, K., Tabe, Y., Chikahisa, T. & Tanuma, T. Water transport and PEFC performance with different interface structure between micro-porous layer and catalyst layer. J. Electrochem. Soc. 163, F359–F366 (2016).
Kalidindi, A. R., Taspinar, R., Litster, S. & Kumbur, E. C. A two-phase model for studying the role of microporous layer and catalyst layer interface on polymer electrolyte fuel cell performance. Int. J. Hydrogen Energy 38, 9297–9309 (2013).
Swamy, T., Kumbur, E. C. & Mench, M. M. Characterization of interfacial structure in PEFCs: water storage and contact resistance model. J. Electrochem. Soc. 157, B77–B85 (2010).
Kucernak, A. R. & Toyoda, E. Studying the oxygen reduction and hydrogen oxidation reactions under realistic fuel cell conditions. Electrochem. Commun. 10, 1728–1731 (2008).
Zalitis, C. M., Kramer, D. & Kucernak, A. R. Electrocatalytic performance of fuel cell reactions at low catalyst loading and high mass transport. Phys. Chem. Chem. Phys. 15, 4329–4340 (2013).
Martens, S. et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 392, 274–284 (2018).
Pinaud, B. A., Bonakdarpour, A., Daniel, L., Sharman, J. & Wilkinson, D. P. Key considerations for high current fuel cell catalyst testing in an electrochemical half-cell. J. Electrochem. Soc. 164, F321–F327 (2017).
Inaba, M. et al. Benchmarking high surface area electrocatalysts in a gas diffusion electrode: measurement of oxygen reduction activities under realistic conditions. Energy Environ. Sci. 11, 988–994 (2018).
Acknowledgements
We thank the National Key Research and Development Program of China (2018YFB1502503, 2020YFB1505800), the Guangdong Provincial Key Laboratory of Energy Materials for Electric Power (2018B030322001), the Foundation Research Project of Shenzhen the Natural Science Fund (JCYJ20200109141216566), the Shenzhen Key Laboratory of Hydrogen Energy (ZDSYS2016033110134898) and the Technology Projects for Sustainable Development (KCXFZ202002011010317).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.Y. is employed by SinoHykey Technology Guangzhou Co. Ltd, which manufactures MEAs/fuel cells. The other authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Di-Jia Liu, Alejandro Martinez-Bonastre and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, J., Chen, M., Zhao, Z. et al. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat Energy 6, 475–486 (2021). https://doi.org/10.1038/s41560-021-00824-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00824-7
This article is cited by
-
Electrocatalytic hydrogenation of acetonitrile to ethylamine in acid
Nature Communications (2024)
-
Inter-site structural heterogeneity induction of single atom Fe catalysts for robust oxygen reduction
Nature Communications (2024)
-
Carbon-based supports for the electrocatalysis under industrially relevant conditions
Science China Chemistry (2024)
-
A high-performance electrocatalyst for oxygen reduction derived from copolymer-anchored polyoxometalates
Nano Research (2024)
-
Advanced Electrode Structures for Proton Exchange Membrane Fuel Cells: Current Status and Path Forward
Electrochemical Energy Reviews (2024)