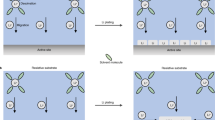
Abstract
Although Li-based batteries have established a dominant role in the current energy-storage landscape, post-Li chemistries (for example, Al or Zn) are emerging as promising candidates for next-generation rechargeable batteries. Electrochemical cells using Al or Zn metal as the negative electrode are of interest for their potential low cost, intrinsic safety and sustainability. Presently, such cells are considered impractical because the reversibility of the metal anode is poor and the amount of charge stored is miniscule. Here we report that electrodes designed to promote strong oxygen-mediated chemical bonding between Al deposits and the substrate enable a fine control of deposition morphology and provide exceptional reversibility (99.6–99.8%). The reversibility is sustained over unusually long cycling times (>3,600 hours) and at areal capacities up to two orders of magnitude higher than previously reported values. We show that these traits result from the elimination of fragile electron transport pathways, and the non-planar deposition of Al via specific metal–substrate chemical bonding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during the current study are available in the article and its Supplementary Information.
References
Josell, D., Wheeler, D., Huber, W. H. & Moffat, T. P. Superconformal electrodeposition in submicron features. Phys. Rev. Lett. 87, 016102 (2001).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345 (2018).
Baker, H. The metal of the future. Nature 42, 537 (1890).
Tikekar, M. D., Choudhury, S., Tu, Z. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016).
Zheng, J. et al. Physical orphaning versus chemical instability: Is dendritic electrodeposition of Li fatal?. ACS Energy Lett. 4, 1349–1355 (2019).
Deng, Y. et al. On the reversibility and fragility of sodium metal electrodes. Adv. Energy Mater. 9, 1901651 (2019).
Shi, F. et al. Strong texturing of lithium metal in batteries. Proc. Natl Acad. Sci. USA 114, 12138–12143 (2017).
Biswal, P., Stalin, S., Kludze, A., Choudhury, S. & Archer, L. A. On the nucleation and early-stage growth of Li electrodeposits. Nano Lett. 19, 8191–8200 (2019).
Bai, P., Li, J., Brushett, F. R. & Bazant, M. Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 9, 3221–3229 (2016).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
Zheng, J. et al. Regulating electrodeposition morphology of lithium: towards commercially relevant secondary Li metal batteries. Chem. Soc. Rev. 49, 2701–2750 (2020).
Zheng, J. & Archer, L. A. Controlling electrochemical growth of metallic zinc electrodes: toward affordable rechargeable energy storage systems. Sci. Adv. 7, eabe0219 (2021).
Lin, M.-C. et al. An ultrafast rechargeable aluminium-ion battery. Nature 520, 324–328 (2015).
Higashi, S., Lee, S. W., Lee, J. S., Takechi, K. & Cui, Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat. Commun. 7, 11801 (2016).
Zhu, C., Usiskin, R. E., Yu, Y. & Maier, J. The nanoscale circuitry of battery electrodes. Science 358, eaao2808 (2017).
Reid, J. Copper electrodeposition: principles and recent progress. Jpn J. Appl. Phys. 40, 2650 (2001).
Elia, G. A. et al. An overview and future perspectives of aluminum batteries. Adv. Mater. 28, 7564–7579 (2016).
Jayaprakash, N., Das, S. K. & Archer, L. A. The rechargeable aluminum-ion battery. Chem. Commun. 47, 12610–12612 (2011).
Faegh, E., Ng, B., Hayman, D. & Mustain, W. E. Practical assessment of the performance of aluminium battery technologies. Nat Energy 6, 21–29 (2021).
Zheng, J. et al. Nonplanar electrode architectures for ultrahigh areal capacity batteries. ACS Energy Lett. 4, 271–275 (2018).
Albertus, P., Babinec, S., Litzelman, S. & Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018).
Levy, N. R. & Ein-Eli, Y. Aluminum-ion battery technology: a rising star or a devastating fall? J. Solid State Electrochem. 24, 2067–2071 (2020).
Chen, H. et al. Oxide film efficiently suppresses dendrite growth in aluminum-ion battery. ACS Appl. Mater. Interfaces 9, 22628–22634 (2017).
Zhao, Q., Zheng, J., Deng, Y. & Archer, L. Regulating the growth of aluminum electrodeposits: towards anode-free Al batteries. J. Mater. Chem. A 8, 23231–23238 (2020).
Chi, S. S., Liu, Y., Song, W. L., Fan, L. Z. & Zhang, Q. Prestoring lithium into stable 3D nickel foam host as dendrite-free lithium metal anode. Adv. Funct. Mater. 27, 1700348 (2017).
Roberts, S. & Dobson, P. J. Evidence for reaction at the Al–SiO2 interface. J. Phys. D 14, L17 (1981).
Bauer, R. S., Bachrach, R. Z. & Brillson, L. J. Au and Al interface reactions with SiO2. Appl. Phys. Lett. 37, 1006–1008 (1980).
Bard A. J. & Faulkner L. R. Electrochemical Methods: Fundamentals and Applications (John Wiley and Sons, 2002).
Yang, H. et al. The rechargeable aluminum battery: opportunities and challenges. Angew. Chem. Int. Ed. 58, 11978–11996 (2019).
Pande, V. & Viswanathan, V. Computational screening of current collectors for enabling anode-free lithium metal Batteries. ACS Energy Lett. 4, 2952–2959 (2019).
So, K. P. et al. Improving the wettability of aluminum on carbon nanotubes. Acta Mater. 59, 3313–3320 (2011).
So, K. P., Biswas, C., Lim, S. C., An, K. H. & Lee, Y. H. Electroplating formation of Al–C covalent bonds on multiwalled carbon nanotubes. Synth. Met. 161, 208–212 (2011).
Choudhury, S. et al. Designing solid–liquid interphases for sodium batteries. Nat. Commun. 8, 898 (2017).
Jadhav, A. L., Xu, J. H. & Messinger, R. J. Quantitative molecular-Level understanding of electrochemical aluminum–ion intercalation into a crystalline battery electrode. ACS Energy Lett. 5, 2842–2848 (2020).
Zhao, Q. et al. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 4, eaau8131 (2018).
Zheng, J. et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 366, 645 (2019).
Wang, Z. et al. A metal–organic framework host for highly reversible dendrite-free zinc metal anodes. Joule 3, 1289–1300 (2019).
Zheng, J. et al. Spontaneous and field-induced crystallographic reorientation of metal electrodeposits at battery anodes. Sci. Adv. 6, eabb1122 (2020).
Vaughan, J. & Dreisinger, D. Electrodeposition of aluminum from aluminum chloride–trihexyl(tetradecyl)phosphonium chloride. J. Electrochem. Soc. 155, D68–D72 (2008).
Barr, T. L. & Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A 13, 1239–1246 (1995).
Paranthaman, P. M. A high performance hybrid battery based on aluminum anode and LiFePO4 cathode. Chem. Commun. 52, 1713–1716 (2016).
Zhang, X., Tang, Y., Zhang, F. & Lee, C. S. A novel aluminum–graphite dual-ion battery. Adv. Energy Mater. 6, 1502588 (2016).
Wang, S. et al. A novel aluminum-ion battery: Al/AlCl3-[EMIm]Cl/Ni3S2@graphene. Adv. Energy Mater. 6, 1600137 (2016).
Chen, H. et al. A defect-free principle for advanced graphene cathode of aluminum-ion battery. Adv. Mater. 29, 1605958 (2017).
Chen, H. et al. Ultrafast all-climate aluminum–graphene battery with quarter-million cycle life. Sci. Adv. 3, eaao7233 (2017).
Liu, Z. et al. Carbon nanoscrolls for aluminum battery. ACS Nano 12, 8456–8466 (2018).
Zhang, E. et al. A novel aluminum dual-ion battery. Energy Storage Mater. 11, 91–99 (2018).
Wu, F., Zhu, N., Bai, Y., Gao, Y. & Wu, C. An interface-reconstruction effect for rechargeable aluminum battery in ionic liquid electrolyte to enhance cycling performances. Green Energy Environ. 3, 71–77 (2018).
Zhang, E. et al. Unzipped carbon nanotubes for aluminum battery. Energy Storage Mater. 23, 72–78 (2019).
Xu, H. et al. Low-cost AlCl3/Et3NHCl electrolyte for high-performance aluminum-ion battery. Energy Storage Mater. 17, 38–45 (2019).
Tan, B. et al. Synthesis of RGO-supported layered MoS2 with enhanced electrochemical performance for aluminum ion batteries. J. Alloy Compd 841, 155732 (2020).
Acknowledgements
We thank F. Zhu, L. Luo, R. Luo, M. Pfeifer and X. Ren for valuable discussions. This work was supported as part of the Center for Mesoscale Transport Properties, an Energy Frontier Research Center supported by the US Department of Energy, Office of Science, Basic Energy Sciences, under award no. DE-SC0012673. This work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the NSF MRSEC program (DMR-1719875). The pouch cell assembly line used for the anode-free electrodes with a 9 cm2 area was supported under award 75039 from the New York State Energy Research and Development Authority (NYSERDA) and award 76890 from the New York State Department of Economic Development (DED), which were provided as matching funds to the Center for Mesoscale Transport Properties under award no. DE-SC0012673. E.S.T. acknowledges support as the William and Jane Knapp Chair of Energy and the Environment.
Author information
Authors and Affiliations
Contributions
L.A.A. directed the research. J.Z. and L.A.A. conceived and designed this work. J.Z. and L.A.A. wrote the paper with inputs from other authors. J.Z. and T.T. performed the electrodeposition, electrochemical measurements and structure characterizations. J.Z., Q.Z., G.W. and D.C.B. assembled the pouch cells. D.C.B. and K.R.T. collected and interpreted the XPS data with input from A.C.M.; Q.Z., J.Y., X.L., Y.D. and J.S. analysed the data. All the authors contributed to the review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Electrochemical plating/stripping behavior of Al metal on nonplanar nickel foam substrate.
a, Coulombic efficiency obtained at 0.8 mAh, 4 mA/cm2. Voltage profiles of Al plating/stripping: (b) 0.8 mAh, 4 mA/cm2;(c) 3.2 mAh, 1.6 mAh/cm2 and (d) 8.0 mAh, 1.6 mA/cm2. The results mean that the improvement made by using a nonplanar, inert architecture is very limited, particularly at practical capacities, that is 3.2 and 8 mAh/cm2.
Extended Data Fig. 2 XPS spectrum of interwoven carbon fibers.
a, Pristine, (b) after exposure to IL+AlCl3 electrolyte, (c) after exposure to dimethyl carbonate as a negative control. Peak assignments: 284.8 eV (C-C, C-H), 286 eV (C-O), 287.5–288 eV (C=O, O-C-O), 289 eV (O=C-O). The intensities at 286 and 287.5 ~ 288 eV suggest that the exposure to IL+AlCl3 notably increases the level of oxygen enrichment on carbon fibers.
Extended Data Fig. 3 SEM images and EDS mapping of Al deposition morphology obtained using a sequential, two-step protocol.
In Step I, a small capacity (0.05 mAh/cm2) of Al is deposited at fixed overpotential, η values (for example, η = 0.05, 0.3, 1.0, 2.0, 3.0 V); In Step II, a greater areal capacity (that is 0.45 mAh/cm2) of Al is galvanostatically deposited at a current density (that is 4 mA/cm2). SEM images of the Al deposition morphology obtained using this protocol, with the η value equal to (a) 0.05 V, (b) 0.3 V, (c) 1.0 V, (d) 2.0 V, and (e) 3.0 V. f–o, the corresponding EDS mapping results. See Supplementary Note 1 for a detailed discussion.
Extended Data Fig. 4. XPS spectrum of Al electrodeposits on carbon fibers, stainless steel and nickel foam.
XPS of Al deposited on (a)–(c) carbon fibers, (d)–(f) stainless steel, and (g)–(i) Ni foam. (a)(d)(g) C 1 s spectra; (b)(e)(h) O 1 s spectra; (c)(f)(i) Al 2p spectra. Upper panels and lower panels show spectra before and after Ar+ sputtering, respectively. After sputtering, the Al-O-C bonding was observed on samples where Al was deposited on carbon fibers. On other samples, no characteristic metal-substrate covalent bonding is observable.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Supplementary Note 1, and Supplementary Figures 1–20.
Rights and permissions
About this article
Cite this article
Zheng, J., Bock, D.C., Tang, T. et al. Regulating electrodeposition morphology in high-capacity aluminium and zinc battery anodes using interfacial metal–substrate bonding. Nat Energy 6, 398–406 (2021). https://doi.org/10.1038/s41560-021-00797-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00797-7
This article is cited by
-
Progress and challenges of electrolyte modulation in aqueous zinc-ion batteries
Rare Metals (2024)
-
Surficial modification enabling planar Al growth toward dendrite-free metal anodes for rechargeable aluminum batteries
Science China Chemistry (2024)
-
Coordination modulation of hydrated zinc ions to enhance redox reversibility of zinc batteries
Nature Communications (2023)
-
MXene chemistry, electrochemistry and energy storage applications
Nature Reviews Chemistry (2022)
-
Lanthanum nitrate as aqueous electrolyte additive for favourable zinc metal electrodeposition
Nature Communications (2022)