Abstract
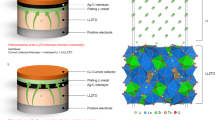
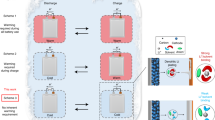
Stable operation of rechargeable lithium-based batteries at low temperatures is important for cold-climate applications, but is plagued by dendritic Li plating and unstable solid–electrolyte interphase (SEI). Here, we report on high-performance Li metal batteries under low-temperature and high-rate-charging conditions. The high performance is achieved by using a self-assembled monolayer of electrochemically active molecules on current collectors that regulates the nanostructure and composition of the SEI and deposition morphology of Li metal anodes. A multilayer SEI that contains a lithium fluoride-rich inner phase and amorphous outer layer effectively seals the Li surface, in contrast to the conventional SEI, which is non-passive at low temperatures. Consequently, galvanic Li corrosion and self-discharge are suppressed, stable Li deposition is achieved from −60 °C to 45 °C, and a Li | LiCoO2 cell with a capacity of 2.0 mAh cm−2 displays a 200-cycle life at −15 °C with a recharge time of 45 min.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All relevant data are included in the paper and its Supplementary Information.
References
Tarascon, J. -M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Nagasubramanian, G. Electrical characteristics of 18650 Li-ion cells at low temperatures. J. Appl. Electrochem. 31, 99–104 (2001).
Zhang, S. S., Xu, K. & Jow, T. R. The low temperature performance of Li-ion batteries. J. Power Sources 115, 137–140 (2003).
Lin, H.-p et al. Low-temperature behavior of Li-ion cells. Electrochem. Solid-State Lett. 4, A71 (2002).
Petzl, M., Kasper, M. & Danzer, M. A. Lithium plating in a commercial lithium-ion battery – a low-temperature aging study. J. Power Sources 275, 799–807 (2015).
Fleischhammer, M., Waldmann, T., Bisle, G., Hogg, B. -I. & Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 274, 432–439 (2015).
Ratnakumar, B. V. et al. Lithium ion batteries for Mars exploration missions. Electrochim. Acta 45, 1513–1517 (2000).
Rodrigues, M.-T. F. et al. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2, 17108 (2017).
Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Peled, E. & Menkin, S. Review—SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Scrosati, B. & Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 195, 2419–2430 (2010).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Wang, C., Appleby, A. J. & Little, F. E. Low-temperature characterization of lithium-ion carbon anodes via microperturbation measurement. J. Electrochem. Soc. 149, A754 (2002).
Zhang, S. S., Xu, K. & Jow, T. R. A new approach toward improved low temperature performance of Li-ion battery. Electrochem. Commun. 4, 928–932 (2002).
Smart, M. C. et al. Improved performance of lithium-ion cells with the use of fluorinated carbonate-based electrolytes. J. Power Sources 119–121, 359–367 (2003).
Smart, M. C. et al. Irreversible capacities of graphite in low-temperature electrolytes for lithium-ion batteries. J. Electrochem. Soc. 146, 3963 (1999).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Tung, S.-O., Ho, S., Yang, M., Zhang, R. & Kotov, N. A. A dendrite-suppressing composite ion conductor from aramid nanofibres. Nat. Commun. 6, 6152 (2015).
Choudhury, S., Mangal, R., Agrawal, A. & Archer, L. A. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat. Commun. 6, 10101 (2015).
Liu, K. et al. Lithium metal anodes with an adaptive “solid-liquid” interfacial protective layer. J. Am. Chem. Soc. 139, 4815–4820 (2017).
Kim, M. S. et al. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018).
Gao, Y. et al. Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 139, 15288–15291 (2017).
Gao, Y. et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019).
Liang, X. et al. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 6, 17119 (2017).
Dudney, N. J. Addition of a thin-film inorganic solid electrolyte (Lipon) as a protective film in lithium batteries with a liquid electrolyte. J. Power Sources 89, 176–179 (2000).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Basile, A., Bhatt, A. I. & O’Mullane, A. P. Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 7, 11794 (2016).
Zeng, Z. et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 3, 674–681 (2018).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Wei, S. et al. Stabilizing electrochemical interfaces in viscoelastic liquid electrolytes. Sci. Adv. 4, eaao6243 (2018).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Yang, C.-P., Yin, Y.-X., Zhang, S.-F., Li, N.-W. & Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 8058 (2015).
Li, G. et al. Stable metal battery anodes enabled by polyethylenimine sponge hosts by way of electrokinetic effects. Nat. Energy 3, 1076–1083 (2018).
Zhang, R. et al. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 56, 7764–7768 (2017).
Rustomji, C. S. et al. Liquefied gas electrolytes for electrochemical energy storage devices. Science 356, eaal4263 (2017).
Yang, Y. et al. High-efficiency lithium-metal anode enabled by liquefied gas electrolytes. Joule 3, 1986–2000 (2019).
Plichta, E. J. & Behl, W. K. A low-temperature electrolyte for lithium and lithium-ion batteries. J. Power Sources 88, 192–196 (2000).
Uvdal, K., Bodö, P. & Liedberg, B. ʟ-cysteine adsorbed on gold and copper: An X-ray photoelectron spectroscopy study. J. Colloid Interface Sci. 149, 162–173 (1992).
Caprioli, F., Decker, F., Marrani, A. G., Beccari, M. & Di Castro, V. Copper protection by self-assembled monolayers of aromatic thiols in alkaline solutions. Phys. Chem. Chem. Phys. 12, 9230–9238 (2010).
Mandler, D. & Turyan, I. Applications of self-assembled monolayers in electroanalytical chemistry. Electroanalysis 8, 207–213 (1996).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 358, 506–510 (2017).
Wang, F. et al. Chemical distribution and bonding of lithium in intercalated graphite: Identification with optimized electron energy loss spectroscopy. ACS Nano 5, 1190–1197 (2011).
Chen, S. et al. Functional organosulfide electrolyte promotes an alternate reaction pathway to achieve high performance in lithium-sulfur batteries. Angew. Chem. Int. Ed. 55, 4231–4235 (2016).
Ding, F. et al. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J. Electrochem. Soc. 160, A1894–A1901 (2013).
Lin, D. et al. Fast galvanic lithium corrosion involving a Kirkendall-type mechanism. Nat. Chem. 11, 382–389 (2019).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy, through the Advanced Battery Materials Research (BMR) Program (Battery500 Consortium) award no. DE-EE0008198. T.R. and A.T.N. acknowledge the provision of computing resources on Bebop, a high-performance computing cluster operated by the Laboratory Computing Resource Center at Argonne National Laboratory. We thank S. Zheng for discussion on the performance of batteries at low temperatures.
Author information
Authors and Affiliations
Contributions
Y.G. and Donghai Wang conceived and designed the experiments. Y.G., T.C., and S.L. prepared the materials and electrodes. T.R. and A.T.N. designed and conducted the electrochemical simulation of the low-temperature SEI. K.W. and H.W. performed the TEM experiments. Y.G. and Daiwei Wang conducted the electrochemical tests. All authors discussed and analysed the data. Y.G. and Donghai Wang prepared the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figs. 1–51, discussion, Tables 1–9 and refs. 1–7.
Rights and permissions
About this article
Cite this article
Gao, Y., Rojas, T., Wang, K. et al. Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface. Nat Energy 5, 534–542 (2020). https://doi.org/10.1038/s41560-020-0640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0640-7
This article is cited by
-
Ligand-channel-enabled ultrafast Li-ion conduction
Nature (2024)
-
Resting restores performance of discharged lithium-metal batteries
Nature (2024)
-
Electrolyte Design for Low-Temperature Li-Metal Batteries: Challenges and Prospects
Nano-Micro Letters (2024)
-
Machine-learning-assisted design of a binary descriptor to decipher electronic and structural effects on sulfur reduction kinetics
Nature Catalysis (2023)
-
A monofluoride ether-based electrolyte solution for fast-charging and low-temperature non-aqueous lithium metal batteries
Nature Communications (2023)