Abstract
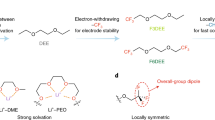
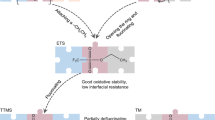
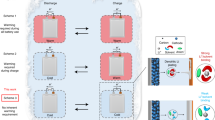
Electrolyte engineering is critical for developing Li metal batteries. While recent works improved Li metal cyclability, a methodology for rational electrolyte design remains lacking. Herein, we propose a design strategy for electrolytes that enable anode-free Li metal batteries with single-solvent single-salt formations at standard concentrations. Rational incorporation of –CF2– units yields fluorinated 1,4-dimethoxylbutane as the electrolyte solvent. Paired with 1 M lithium bis(fluorosulfonyl)imide, this electrolyte possesses unique Li–F binding and high anion/solvent ratio in the solvation sheath, leading to excellent compatibility with both Li metal anodes (Coulombic efficiency ~ 99.52% and fast activation within five cycles) and high-voltage cathodes (~6 V stability). Fifty-μm-thick Li|NMC batteries retain 90% capacity after 420 cycles with an average Coulombic efficiency of 99.98%. Industrial anode-free pouch cells achieve ~325 Wh kg−1 single-cell energy density and 80% capacity retention after 100 cycles. Our design concept for electrolytes provides a promising path to high-energy, long-cycling Li metal batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are included in the paper and its Supplementary Information.
Code availability
The Python script for analysing the Li+ solvation structure is available at https://github.com/xianshine/LiSolvationStructure.git.
References
Press release: The Nobel Prize in Chemistry 2019. The Nobel Prize https://www.nobelprize.org/prizes/chemistry/2019/press-release/ (2019).
Battery revolution to evolution. Nat. Energy 4, 893 (2019).
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Albertus, P., Babinec, S., Litzelman, S. & Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Liu, B., Zhang, J. & Xu, W. Advancing lithium metal batteries. Joule 2, 833–845 (2018).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Cao, Y., Li, M., Lu, J., Liu, J. & Amine, K. Bridging the academic and industrial metrics for next-generation practical batteries. Nat. Nanotechnol. 14, 200–207 (2019).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Liu, Y. et al. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 9, 3656 (2018).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Fan, X. et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 4, 882–890 (2019).
Yang, Y. et al. High-efficiency lithium-metal anode enabled by liquefied gas electrolytes. Joule 3, 1986–2000 (2019).
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Fan, X. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Ren, X. et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 4, 1877–1892 (2018).
Niu, C. et al. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 4, 551–559 (2019).
Chen, S. et al. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2, 1548–1558 (2018).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Amanchukwu, C. V. et al. A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability. J. Am. Chem. Soc. 142, 7393–7403 (2020).
Jiao, S. et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 3, 739–746 (2018).
Li, N., Leng, Y., Hickner, M. A. & Wang, C. Y. Highly stable, anion conductive, comb-shaped copolymers for alkaline fuel cells. J. Am. Chem. Soc. 135, 10124–10133 (2013).
Zhang, X., Sheng, L., Higashihara, T. & Ueda, M. Polymer electrolyte membranes based on poly(m-phenylene)s with sulfonic acid via long alkyl side chains. Polym. Chem. 4, 1235–1242 (2013).
Li, T., Zhang, X.-Q., Shi, P. & Zhang, Q. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule 3, 2647–2661 (2019).
Aspern, N., Röschenthaler, G.-V., Winter, M. & Cekic‐Laskovic, I. Fluorine and lithium: ideal partners for high‐performance rechargeable battery electrolytes. Angew. Chem. Int. Ed. 58, 15978–16000 (2019).
Zhang, Z. et al. Fluorinated electrolytes for 5 V lithium-ion battery chemistry. Energy Environ. Sci. 6, 1806–1810 (2013).
Zeng, Z. et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 3, 674–681 (2018).
Suo, L. et al. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl Acad. Sci. USA 115, 1156–1161 (2018).
Xue, W. et al. FSI-inspired solvent and “full fluorosulfonyl” electrolyte for 4 V class lithium-metal batteries. Energy Environ. Sci. 13, 212–220 (2020).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J.-G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Liu, Y. et al. An artificial solid electrolyte interphase with high Li-ion conductivity, mechanical strength, and flexibility for stable lithium metal anodes. Adv. Mater. 29, 1605531 (2017).
Zhang, J. G. Anode-less. Nat. Energy 4, 637–638 (2019).
Weber, R. et al. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 4, 683–689 (2019).
Genovese, M. et al. Hot formation for improved low temperature cycling of anode-free lithium metal batteries. J. Electrochem. Soc. 166, A3342–A3347 (2019).
Nanda, S., Gupta, A. & Manthiram, A. Anode‐free full cells: a pathway to high‐energy density lithium‐metal batteries. Adv. Energy Mater. 2000804 (2020). https://doi.org/10.1002/aenm.202000804
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 358, 506–510 (2017).
Huang, W., Wang, H., Boyle, D. T., Li, Y. & Cui, Y. Resolving nanoscopic and mesoscopic heterogeneity of fluorinated species in battery solid-electrolyte interphases by cryogenic electron microscopy. ACS Energy Lett. 5, 1128–1135 (2020).
Wang, J. et al. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019).
Li, Y. et al. Correlating structure and function of battery interphases at atomic resolution using cryoelectron microscopy. Joule 2, 2167–2177 (2018).
Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019).
Yu, Z. et al. A dynamic, electrolyte-blocking, and single-ion-conductive network for stable lithium-metal anodes. Joule 3, 2761–2776 (2019).
Dou, J.-H. et al. Organic semiconducting alloys with tunable energy levels. J. Am. Chem. Soc. 141, 6561–6568 (2019).
Ren, X. et al. High-concentration ether electrolytes for stable high-voltage lithium metal batteries. ACS Energy Lett. 4, 896–902 (2019).
Mackanic, D. G. et al. Crosslinked poly(tetrahydrofuran) as a loosely coordinating polymer electrolyte. Adv. Energy Mater. 8, 1800703 (2018).
Henderson, W. A., Brooks, N. R. & Smyrl, W. H. Polymeric [Li(NO3)(monoglyme)]n. Acta Crystallogr. E 58, m500–m501 (2002).
Wang, Z. et al. An anion‐tuned solid electrolyte interphase with fast ion transfer kinetics for stable lithium anodes. Adv. Energy Mater. 10, 1903843 (2020).
Zhang, C. et al. Chelate effects in glyme/lithium bis(trifluoromethanesulfonyl)amide solvate ionic liquids, part 2: Importance of solvate-structure stability for electrolytes of lithium batteries. J. Phys. Chem. C 118, 17362–17373 (2014).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Sambasivarao, S. V. & Acevedo, O. Development of OPLS-AA force field parameters for 68 unique ionic liquids. J. Chem. Theory Comput. 5, 1038–1050 (2009).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Michaud-Agrawal, N., Denning, E. J., Woolf, T. B. & Beckstein, O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Burla, M. C. et al. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 48, 306–309 (2015).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Acknowledgements
This work is supported by the US Department of Energy, under the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies, Battery Materials Research (BMR) Program, and by the Battery 500 Consortium. Part of this work was performed at the Stanford Nano Shared Facilities, supported by the National Science Foundation under award ECCS-1542152. Z.Y. thanks X. Xu from Hunan Li-Fun Technology for fabricating pouch cells, Beijing Golden Feather New Energy Technology for providing LiFSI and Z. Yao for discussion on the DFT calculations. All authors thank K. Zaghib from Hydro-Québec for preparing and providing the thin Li metal foils. D.G.M. acknowledges support by the National Science Foundation Graduate Research Fellowship Program under grant no. (DGE‐114747). C.V.A. acknowledges the TomKat Center Postdoctoral Fellowship in Sustainable Energy for funding support.
Author information
Authors and Affiliations
Contributions
Z.Y., H.W., Y.C. and Z.B. conceived the idea. Z.Y. and H.W. designed the experiments. Y.C. and Z.B. directed the project. Z.Y. performed the syntheses, material characterizations, DFT calculations, electrochemical measurements and coin-cell tests. H.W. performed the XPS measurements, pouch-cell fabrication and tests, and coin-cell tests. X.K. and J.Q. conducted the MD simulations and rationales. William Huang performed the cryo-TEM and cryogenic energy-dispersive X-ray spectroscopy measurements. Y.T., William Huang and E.G.L. performed the SEM experiments. K.W. and X.W. helped with the single-crystal measurement and structure refinement. D.G.M. and C.V.A. collected the 7Li-NMR spectra. Wenxiao Huang helped with the pouch-cell fabrication and tests. S.C. measured the viscosities. Y.Z. collected the ultraviolet–visible spectra. S.T.H. measured the water contents. Y.M. helped with the syntheses. All authors discussed and analysed the data. Z.Y., H.W., Y.C. and Z.B. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
This work has been filed as US Provisional Patent Application No. 62/928,638.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Figs. 1–53 and refs. 1–16.
Crystallographic Data 1
LiTf-FDMB single crystal.
Crystallographic Data 2
LiTf-DMB single crystal.
Supplementary Video
Flammability test of conventional carbonate electrolyte (left, 1 M LiPF6 in EC/EMC) and FDMB (right). It can be observed that conventional carbonate electrolyte was flammable immediately after touching the fire of the lighter; however, FDMB can tolerate the direct touch of fire for at least three seconds.
Rights and permissions
About this article
Cite this article
Yu, Z., Wang, H., Kong, X. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat Energy 5, 526–533 (2020). https://doi.org/10.1038/s41560-020-0634-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0634-5
This article is cited by
-
Materials descriptors of machine learning to boost development of lithium-ion batteries
Nano Convergence (2024)
-
Hybridizing carbonate and ether at molecular scales for high-energy and high-safety lithium metal batteries
Nature Communications (2024)
-
Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries
Nature Energy (2024)
-
Tuning the solvation structure with salts for stable sodium-metal batteries
Nature Energy (2024)
-
Methylation enables the use of fluorine-free ether electrolytes in high-voltage lithium metal batteries
Nature Chemistry (2024)