Abstract
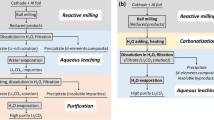
As the consumption of lithium-ion batteries (LIBs) for the transportation and consumer electronic sectors continues to grow, so does the pile of battery waste, with no successful recycling model, as exists for the lead–acid battery. Here, we exhibit a method to recycle LIBs using deep eutectic solvents to extract valuable metals from various chemistries, including lithium cobalt (iii) oxide and lithium nickel manganese cobalt oxide. For the metal extraction from lithium cobalt (iii) oxide, leaching efficiencies of ≥90% were obtained for both cobalt and lithium. It was also found that other battery components, such as aluminium foil and polyvinylidene fluoride binder, can be recovered separately. Deep eutectic solvents could provide a green alternative to conventional methods of LIB recycling and reclaiming strategically important metals, which remain crucial to meet the demand of the exponentially increasing LIB production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots and tables within this paper and its Supplementary Information files, as well as the other findings of this study, are available from the corresponding author upon reasonable request.
References
Zheng, X. et al. A mini-review on metal recycling from spent lithium ion batteries. Engineering 4, 361–370 (2018).
Zeng, X., Li, J. & Singh, N. Recycling of spent lithium-ion battery: a critical review. Crit. Rev. Env. Sci. Tech. 44, 1129–1165 (2014).
Frankel, T. C. The cobalt pipeline: tracing the path from deadly hand-dug mines in Congo to consumers' phones and laptops. The Washington Post (30 September 2016); https://www.washingtonpost.com/graphics/business/batteries/congo-cobalt-mining-for-lithium-ion-battery/?noredirect=on
Peters, J. F., Baumann, M., Zimmermann, B., Braun, J. & Weil, M. The environmental impact of Li-ion batteries and the role of key parameters—a review. Renew. Sustain. Energy Rev. 67, 491–506 (2017).
Zhang, P., Yokoyama, T., Itabashi, O., Suzuki, T. M. & Inoue, K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47, 259–271 (1998).
Li, J., Wang, G. & Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 302, 97–104 (2016).
Guo, Y. et al. Improved extraction of cobalt and lithium by reductive acid from spent lithium-ion batteries via mechanical activation process. J. Mater. Sci. 53, 13790–13800 (2018).
Chagnes, A. & Swiatowska, J. Lithium Process Chemistry: Resources, Extraction, Batteries, and Recycling (Elsevier, 2015).
Huang, B., Pan, Z., Su, X. & An, L. Recycling of lithium-ion batteries: recent advances and perspectives. J. Power Sources 399, 274–286 (2018).
Chen, W.-S. & Ho, H.-J. Recovery of valuable metals from lithium-ion batteries NMC cathode waste materials by hydrometallurgical methods. Metals 8, 321 (2018).
Sun, C., Xu, L., Chen, X., Qiu, T. & Zhou, T. Sustainable recovery of valuable metals from spent lithium-ion batteries using DL-malic acid: leaching and kinetics aspect. Waste Manage. Res. 36, 113–120 (2018).
Yao, L., Yao, H., Xi, G. & Feng, Y. Recycling and synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries using d,l-malic acid. RSC Adv. 6, 17947–17954 (2016).
Yao, Y. et al. Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review. ACS Sustain. Chem. Eng. 6, 13611–13627 (2018).
Sun, L. & Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manage. 32, 1575–1582 (2012).
Albler, F. J., Bica, K., Foreman, M. R. S. J., Holgersson, S. & Tyumentsev, M. S. A comparison of two methods of recovering cobalt from a deep eutectic solvent: implications for battery recycling. J. Clean. Prod. 167, 806–814 (2017).
Di Marino, D., Shalaby, M., Kriescher, S. & Wessling, M. Corrosion of metal electrodes in deep eutectic solvents. Electrochem. Commun. 90, 101–105 (2018).
Foreman, M. R. S. Progress towards a process for the recycling of nickel metal hydride electric cells using a deep eutectic solvent. Cogent Chem. 2, 1139289 (2016).
Abbott, A. P., Capper, G., Davies, D. L., McKenzie, K. J. & Obi, S. U. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 51, 1280–1282 (2006).
Zhang, Q., De Oliveira Vigier, K., Royer, S. & Jérôme, F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 41, 7108–7146 (2012).
Zhao, B.-Y. et al. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustain. Chem. Eng. 3, 2746–2755 (2015).
García, G., Aparicio, S., Ullah, R. & Atilhan, M. Deep eutectic solvents: physicochemical properties and gas separation applications. Energy Fuels 29, 2616–2644 (2015).
Radošević, K. et al. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 112, 46–53 (2015).
Millia, L. et al. Bio-inspired choline chloride-based deep eutectic solvents as electrolytes for lithium-ion batteries. Solid State Ion. 323, 44–48 (2018).
Tang, B., Zhang, H. & Row, K. H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples: other techniques. J. Sep. Sci. 38, 1053–1064 (2015).
Smith, E. L., Abbott, A. P. & Ryder, K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 114, 11060–11082 (2014).
Contestabile, M., Panero, S. & Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 92, 65–69 (2001).
Pegoretti, V. C. B., Dixini, P. V. M., Smecellato, P. C., Biaggio, S. R. & Freitas, M. B. J. G. Thermal synthesis, characterization and electrochemical study of high-temperature (HT) LiCoO2 obtained from Co(OH)2 recycled of spent lithium ion batteries. Mater. Res. Bull. 86, 5–9 (2017).
Chen, X. et al. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep. Purif. Technol. 144, 197–205 (2015).
Joulié, M., Laucournet, R. & Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 247, 551–555 (2014).
Coleman, J. S. Chloride complexes of cobalt(II) in anion and cation exchangers. J. Inorg. Nucl. Chem. 28, 2371–2378 (1966).
Hsieh, Y.-T., Lai, M.-C., Huang, H.-L. & Sun, I.-W. Speciation of cobalt-chloride-based ionic liquids and electrodeposition of Co wires. Electrochim. Acta 117, 217–223 (2014).
Wellens, S. et al. Dissolution of metal oxides in an acid-saturated ionic liquid solution and investigation of the back-extraction behaviour to the aqueous phase. Hydrometallurgy 144–145, 27–33 (2014).
Wellens, S., Thijs, B. & Binnemans, K. An environmentally friendlier approach to hydrometallurgy: highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem. 14, 1657–1665 (2012).
Toshima, N. & Yonezawa, T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J. Chem. 22, 1179–1201 (1998).
Luo, C., Zhang, Y., Zeng, X., Zeng, Y. & Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 288, 444–448 (2005).
Huheey, J. E., Keiter, E. A., Keiter, R. L. & Medhi, O. K. Inorganic Chemistry: Principles of Structure and Reactivity (Pearson Education, 2006).
Du, H. et al. Morphology control of CoCO3 crystals and their conversion to mesoporous Co3O4 for alkaline rechargeable batteries application. CrystEngComm 15, 6101–6109 (2013).
González-López, J., Fernández-González, Á. & Jiménez, A. Precipitation behaviour in the system Ca2+-Co2+-CO3 2−-H2O at ambient conditions—amorphous phases and CaCO3 polymorphs. Chem. Geol. 482, 91–100 (2018).
Barber, D. M., Malone, P. G. & Larson, R. J. The effect of cobalt ion on nucleation of calcium-carbonate polymorphs. Chem. Geol. 16, 239–241 (1975).
Katsikopoulos, D., Fernández-González, Á., Prieto, A. C. & Prieto, M. Co-crystallization of Co(ii) with calcite: implications for the mobility of cobalt in aqueous environments. Chem. Geol. 254, 87–100 (2008).
Xia, X. et al. Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv. 2, 1835–1841 (2012).
Cheng, J. P., Chen, X., Ma, R., Liu, F. & Zhang, X. B. A facile method to fabricate porous Co3O4 hierarchical microspheres. Mater. Charact. 62, 775–780 (2011).
Biesinger, M. C. et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 257, 2717–2730 (2011).
Khassin, A. A. et al. Metal–support interactions in cobalt–aluminum co-precipitated catalysts: XPS and CO adsorption studies. J. Mol. Catal. Chem. 175, 189–204 (2001).
Moulder, J. F., Stickle, W. F., Sobol, P. E. & Moben, K. D. Handbook of X-Ray Photoelectron Spectroscopy (Perkin-Elmer Corporation, 1992).
Stoch, J. & Gablankowska-Kukucz, J. The effect of carbonate contaminations on the XPS O 1s band structure in metal oxides. Surf. Interface Anal. 17, 165–167 (1991).
Zeng, X., Li, J. & Singh, N. Recycling of spent lithium-ion battery: a critical review. Crit. Rev. Environ. Sci. Technol. 44, 1129–1165 (2014).
Wanger, T. C. The lithium future—resources, recycling, and the environment: the lithium future. Conserv. Lett. 4, 202–206 (2011).
Harifi-Mood, A. R. & Buchner, R. Density, viscosity, and conductivity of choline chloride + ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide. J. Mol. Liq. 225, 689–695 (2017).
Wang, M.-M., Zhang, C.-C. & Zhang, F.-S. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach. Waste Manage. 51, 239–244 (2016).
Fan, B., Chen, X., Zhou, T., Zhang, J. & Xu, B. A sustainable process for the recovery of valuable metals from spent lithium-ion batteries. Waste Manage. Res. 34, 474–481 (2016).
Bhargava, S., Pownceby, M. & Ram, R. Hydrometallurgy (MDPI, 2017).
Jo, H., Jo, H., Rha, S. & Lee, P.-K. Direct aqueous mineral carbonation of waste slate using ammonium salt solutions. Metals 5, 2413–2427 (2015).
Acknowledgements
The authors thank A. Kabbani and A. Puthirath for useful discussion, and L. Alexander for assistance with the NMC dissolution experiments. M.K.T. acknowledges the National Science Foundation for continued support and funding. This study is based on work supported by the National Science Foundation Graduate Research Fellowship Program under grant number 1450681. Any opinions, findings, conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
M.-T.F.R. conceived of the experimental design. M.K.T. performed the experiments and, alongside M.-T.F.R., co-wrote the paper and analysed the data. K.K. assisted in figure creation, as well as XPS and electrochemical experimentation and analysis. P.M.A. and G.B. conceived of and contributed to the overall project planning.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary Tables 1–3, Supplementary Figures 1–6, Supplementary References
Rights and permissions
About this article
Cite this article
Tran, M.K., Rodrigues, MT.F., Kato, K. et al. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy 4, 339–345 (2019). https://doi.org/10.1038/s41560-019-0368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0368-4
This article is cited by
-
Extraction and recycling technologies of cobalt from primary and secondary resources: A comprehensive review
International Journal of Minerals, Metallurgy and Materials (2024)
-
Ionic liquids and deep eutectic solvents in wastewater treatment: recent endeavours
International Journal of Environmental Science and Technology (2024)
-
Engineering classification recycling of spent lithium-ion batteries through pretreatment: a comprehensive review from laboratory to scale-up application
Rare Metals (2024)
-
Towards Greener Recycling: Direct Repair of Cathode Materials in Spent Lithium-Ion Batteries
Electrochemical Energy Reviews (2024)
-
Recent advancements in hydrometallurgical recycling technologies of spent lithium-ion battery cathode materials
Rare Metals (2024)