Abstract
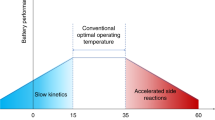
The key to enabling long-term cycling stability of high-voltage lithium (Li) metal batteries is the development of functional electrolytes that are stable against both Li anodes and high-voltage (above 4 V versus Li/Li+) cathodes. Due to their limited oxidative stability ( <4 V), ethers have so far been excluded from being used in high-voltage batteries, in spite of their superior reductive stability against Li metal compared to conventional carbonate electrolytes. Here, we design a concentrated dual-salt/ether electrolyte that induces the formation of stable interfacial layers on both a high-voltage LiNi1/3Mn1/3Co1/3O2 cathode and the Li metal anode, thus realizing a capacity retention of >90% over 300 cycles and ~80% over 500 cycles with a charge cut-off voltage of 4.3 V. This study offers a promising approach to enable ether-based electrolytes for high-voltage Li metal battery applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Cheng, X. B., Zhang, R., Zhao, C. Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 117, 10403–10473 (2017).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotech. 9, 618–623 (2014).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotech. 12, 194–206 (2017).
Zheng, J. M. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Pistoia, G. Nonaqueous batteries with LiClO4‐ethylene carbonate as electrolyte. J. Electrochem. Soc. 118, 153–158 (1971).
Ding, F. et al. Effects of carbonate solvents and lithium salts on morphology and Coulombic efficiency of lithium electrode. J. Electrochem. Soc. 160, A1894–A1901 (2013).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 148, 405–416 (2002).
Basile, A., Bhatt, A. I. & O’Mullane, A. P. Stabilizing lithium metal using ionic liquids for long-lived batteries. Nat. Commun. 7, 11794 (2016).
Girard, G. M. A. et al. Role of Li concentration and the SEI layer in enabling high performance Li metal electrodes using a phosphonium bis(fluorosulfonyl)imide ionic liquid. J. Phys. Chem. C 121, 21087–21095 (2017).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Miao, R. et al. A new ether-based electrolyte for dendrite-free lithium-metal based rechargeable batteries. Sci. Rep. 6, 21771 (2016).
Park, M. S. et al. A highly reversible lithium metal anode. Sci. Rep. 4, 3815 (2014).
Gofer, Y., Ben-Zion, M. & Aurbach, D. Solutions of LiAsF6 in 1,3-dioxolane for secondary lithium batteries. J. Power Sources 39, 163–178 (1992).
Aurbach, D., Zaban, A., Gofer, Y., Abramson, O. & Ben‐Zion, M. Studies of Li anodes in the electrolyte system 2Me-THF/THF/Me-Furan/LiAsF6. J. Electrochem. Soc. 142, 687–696 (1995).
Hayashi, K., Nemoto, Y., Tobishima, S.-I. & Yamaki, J.-I. Mixed solvent electrolyte for high voltage lithium metal secondary cells. Electrochim. Acta 44, 2337–2344 (1999).
Jang, D. H. & Oh, S. M. Electrolyte effects on spinel dissolution and cathodic capacity losses in 4 V Li∕LixMn2O4 rechargeable cells. J. Electrochem. Soc. 144, 3342–3348 (1997).
Yamada, Y. & Yamada, A. Review—Superconcentrated electrolytes for lithium batteries. J. Electrochem. Soc. 162, A2406–A2423 (2015).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun. 6, 6362 (2015).
Miao, R. et al. Novel dual-salts electrolyte solution for dendrite-free lithium-metal based rechargeable batteries with high cycle reversibility. J. Power Sources 271, 291–297 (2014).
Yoshida, K. et al. Oxidative-stability enhancement and charge transport mechanism in glyme-lithium salt equimolar complexes. J. Am. Chem. Soc. 133, 13121–13129 (2011).
Fang, Z. et al. Novel concentrated Li[(FSO2)(n-C4F9SO2)N]-based ether electrolyte for superior stability of metallic lithium anode. ACS Appl. Mater. Interfaces 9, 4282–4289 (2017).
Liu, B. et al. Enhanced cycling stability of rechargeable Li–O2 batteries using high-concentration electrolytes. Adv. Funct. Mater. 26, 605–613 (2016).
Matsumoto, K. et al. Suppression of aluminum corrosion by using high concentration LiTFSI electrolyte. J. Power Sources 231, 234–238 (2013).
Hu, M., Wei, J., Xing, L. & Zhou, Z. Effect of lithium difluoro(oxalate)borate (LiDFOB) additive on the performance of high-voltage lithium-ion batteries. J. Appl. Electrochem. 42, 291–296 (2012).
Chen, K.-H. et al. Dead lithium: mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 5, 11671–11681 (2017).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J.-G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Li, X. et al. Effects of imide-orthoborate dual-salt mixtures in organic carbonate electrolytes on the stability of lithium metal batteries. ACS Appl. Mater. Interfaces 10, 2469–2479 (2018).
Schedlbauer, T. et al. Lithium difluoro(oxalato)borate: A promising salt for lithium metal based secondary batteries? Electrochim. Acta 92, 102–107 (2013).
Zheng, J. et al. Suppressed oxygen extraction and degradation of LiNixMnyCozO2 cathodes at high charge cut-off voltages. Nano Res. 10, 4221–4231 (2017).
Ding, F. et al. Effects of carbonate solvents and lithium salts on morphology and Coulombic efficiency of lithium electrode. J. Electrochem. Soc. 160, A1894–A1901 (2013).
Frisch, M. J. et al. Gaussian09 Revision D.01. (Gaussian Inc., Wallingford CT, 2009).
Boys, S. F. & Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE) through the Advanced Battery Materials Research (BMR) program (Battery500 Consortium) under contract no. DE-AC02-05CH11231. The SEM, EDX, XRD, XPS and computational calculations were conducted in the William R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at PNNL. PNNL is operated by Battelle for the DOE under Contract DE-AC05-76RLO1830. The LiDFOB salt was produced at the US DOE Materials Engineering Research Facility (MERF) and provided by K. Z. Pupek and T. L. Dzwiniel of ANL. The NMC442 electrode was made by the Cell Analysis, Modelling, and Prototyping (CAMP) Facility and provided by B. J. Polzin of ANL. The MERF and CAMP Facilities are fully supported by the DOE Vehicle Technologies Program within the core funding of the Applied Battery Research (ABR) for Transportation Program. This work was performed, in part, at the Center for Nanoscale Materials, a US Department of Energy Office of Science User Facility, and supported by the US Department of Energy, Office of Science, under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
W.X. and J.-G.Z. proposed the research. W.X., S.J., X.R. and R.C. designed the experiments. S.J. and X.R. contributed equally to this work. S.J. and X.R. performed the electrochemical measurements with assistance from R.C. X.R., R.C. and J.Z. conducted the SEM and EDX observations. M.H.E. performed XPS measurements. Y.L. conducted TEM characterizations. D.H. performed IR measurements. W.Z. helped with XRD analysis. R.C. and J.-G.Z. participated in data analyses and discussion. D.M. and N.L. performed molecular dynamics calculations. Q.L. prepared the NMC electrodes. B.D.A. helped with the Li CE calculation with 50 μm Li foil. C.M. analysed the TEM results. S.J., X.R. and W.X. prepared this manuscript with inputs from all other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–21, Supplementary Tables 1–4, Supplementary References
Rights and permissions
About this article
Cite this article
Jiao, S., Ren, X., Cao, R. et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat Energy 3, 739–746 (2018). https://doi.org/10.1038/s41560-018-0199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0199-8
This article is cited by
-
Molecular anchoring of free solvents for high-voltage and high-safety lithium metal batteries
Nature Communications (2024)
-
Methylation enables the use of fluorine-free ether electrolytes in high-voltage lithium metal batteries
Nature Chemistry (2024)
-
A dicarbonate solvent electrolyte for high performance 5 V-Class Lithium-based batteries
Nature Communications (2024)
-
Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries
Nature Communications (2024)
-
Unraveling electrolyte solvation architectures for high-performance lithium-ion batteries
Science China Technological Sciences (2024)