Abstract
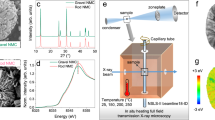
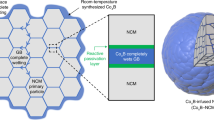
A critical challenge for the commercialization of layer-structured nickel-rich lithium transition metal oxide cathodes for battery applications is their capacity and voltage fading, which originate from the disintegration and lattice phase transition of the cathode particles. The general approach of cathode particle surface modification could partially alleviate the degradation associated with surface processes, but it still fails to resolve this critical barrier. Here, we report that infusing the grain boundaries of cathode secondary particles with a solid electrolyte dramatically enhances the capacity retention and voltage stability of the cathode. We find that the solid electrolyte infused in the boundaries not only acts as a fast channel for lithium-ion transport, it also, more importantly, prevents penetration of the liquid electrolyte into the boundaries, and consequently eliminates the detrimental factors, which include cathode–liquid electrolyte interfacial reactions, intergranular cracking and layered-to-spinel phase transformation. This grain-boundary engineering approach provides design ideas for advanced cathodes for batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Etacheri, V., Marom, R., Elazari, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011).
Zhou, Y.-N. et al. Tuning charge–discharge induced unit cell breathing in layer-structured cathode materials for lithium-ion batteries. Nat. Commun. 5, 5381 (2014).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotech. 12, 194–206 (2017).
Li, W., Song, B. & Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 46, 3006–3059 (2017).
Liu, J. & Sun, X. Elegant design of electrode and electrode/electrolyte interface in lithium-ion batteries by atomic layer deposition. Nanotechnology 26, 024001 (2015).
Manthiram, A., Knight, J. C., Myung, S. T., Oh, S. M. & Sun, Y. K. Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater. 6, 1501010 (2016).
Lee, E.-J. et al. Development of microstrain in aged lithium transition metal oxides. Nano Lett. 14, 4873–4880 (2014).
Liu, H. et al. Intergranular cracking as a major cause of long-term capacity fading of layered cathodes. Nano Lett. 17, 3452–3457 (2017).
Wang, Y. et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 6, 6401 (2015).
Edström, K., Gustafsson, T. & Thomas, J. O. The cathode–electrolyte interface in the Li-ion battery. Electrochim. Acta 50, 397–403 (2004).
Cresce, A. V., Russell, S. M., Baker, D. R., Gaskell, K. J. & Xu, K. In situ and quantitative characterization of solid electrolyte interphases. Nano Lett. 14, 1405–1412 (2014).
Kim, H., Kim, M. G., Jeong, H. Y., Nam, H. & Cho, J. A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2 cathode material: nanoscale surface treatment of primary particles. Nano Lett. 15, 2111–2119 (2015).
Luo, J., Cheng, H. K., Asl, K. M., Kiely, C. J. & Harmer, M. P. The role of a bilayer interfacial phase on liquid metal embrittlement. Science 333, 1730–1733 (2011).
Cho, J., Wang, C. M., Chan, H. M., Rickman, J. M. & Harmer, M. P. Role of segregating dopants on the improved creep resistance of aluminum oxide. Acta Mater. 47, 4197–4207 (1999).
Buban, J. P. et al. Grain boundary strengthening in alumina by rare earth impurities. Science 311, 212–215 (2006).
Shibata, N. et al. Observation of rare-earth segregation in silicon nitride ceramics at subnanometre dimensions. Nature 428, 730–733 (2004).
Appapillai, A. T., Mansour, A. N., Cho, J. & Shao-Horn, Y. Microstructure of LiCoO2 with and without 'AlPO4' nanoparticle coating: combined STEM and XPS studies. Chem. Mater. 19, 5748–5757 (2007).
Lee, Y. et al. Facile formation of a Li3PO4 coating layer during the synthesis of a lithium-rich layered oxide for high-capacity lithium-ion batteries. J. Power Sources 315, 284–293 (2016).
Sun, K. & Dillon, S. J. A mechanism for the improved rate capability of cathodes by lithium phosphate surficial films. Electrochem. Commun. 13, 200–202 (2011).
Li, X. et al. Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 7, 768–778 (2014).
Miller, D. J., Proff, C., Wen, J. G., Abraham, D. P. & Bareño, J. Observation of microstructural evolution in Li battery cathode oxide particles by in situ electron microscopy. Adv. Energy Mater. 3, 1098–1103 (2013).
Bak, S.-M. et al. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 6, 22594–22601 (2014).
Lim, J.-M. et al. Intrinsic origins of crack generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 7, 39669 (2017).
Wang, H., Jang, Y. I., Huang, B., Sadoway, D. R. & Chiang, Y. M. TEM study of electrochemical cycling‐induced damage and disorder in LiCoO2 cathodes for rechargeable lithium batteries. J. Electrochem. Soc. 146, 473–480 (1999).
Kim, J. et al. Controllable solid electrolyte interphase in nickel-rich cathodes by an electrochemical rearrangement for stable lithium-ion batteries. Adv. Mater. 30, 1704309 (2018).
Ryu, H.-H., Park, K.-J., Yoon, C. S. & Sun, Y.-K. Capacity fading of Ni-rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation? Chem. Mater. 30, 1155–1163 (2018).
Yan, P. et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 8, 14101 (2017).
Acknowledgements
We thank R. Liu and Y. Yang from Xiamen University for the DSC test and M. Sui for support on the TEM analysis. This work is supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (DOE) under contract no. DE-AC02-05CH11231, subcontract no. 18769 and no. 6951379 under the Advanced Battery Materials Research program. The microscopic analysis in this work was conducted in the William R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the DOE under contract DE-AC05-76RL01830. Solid-state electrolyte coating by ALD was conducted in the lab of X.S. and is financially supported by the Nature Sciences and Engineering Research Council of Canada Program, Canada Research Chair Program, Canada Foundation for Innovation and the University of Western Ontario. Part of ALD coating was done in BJUT (Y.Z.) under the support of National Natural Science Foundation of China (21676005). P.Y. thanks the National Natural Science Fund for Innovative Research Groups (grant no. 51621003) and the National Key Research and Development Program of China (grant no. 2016YFB0700700).
Author information
Authors and Affiliations
Contributions
C.W., J.Z. and J.-G.Z. initiated this research project. J.Z. and J.L. synthesized the cathode materials. J.L., B.W., X.C., Y.Z. and X.S. carried out the ALD coating. J.Z. and X.C. performed battery tests. P.Y. conducted the TEM and SEM analyses. P.Y., J.Z., C.W. and J.-G.Z. prepared the manuscript with the input from all the other co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–16
Rights and permissions
About this article
Cite this article
Yan, P., Zheng, J., Liu, J. et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat Energy 3, 600–605 (2018). https://doi.org/10.1038/s41560-018-0191-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0191-3
This article is cited by
-
Tuning the solvation structure with salts for stable sodium-metal batteries
Nature Energy (2024)
-
Re-evaluation of battery-grade lithium purity toward sustainable batteries
Nature Communications (2024)
-
Insight into structural degradation of NCMs under extreme fast charging process
Rare Metals (2024)
-
Surface-targeted functionalization of nickel-rich cathodes through synergistic slurry additive approach with multi-level impact using minimal quantity
Nano Research (2024)
-
Suppressing strain propagation in ultrahigh-Ni cathodes during fast charging via epitaxial entropy-assisted coating
Nature Energy (2024)