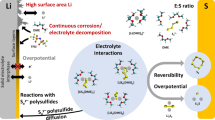
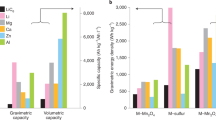
Abstract
Batteries including lithium-ion, lead–acid, redox-flow and liquid-metal batteries show promise for grid-scale storage, but they are still far from meeting the grid's storage needs such as low cost, long cycle life, reliable safety and reasonable energy density for cost and footprint reduction. Here, we report a rechargeable manganese–hydrogen battery, where the cathode is cycled between soluble Mn2+ and solid MnO2 with a two-electron reaction, and the anode is cycled between H2 gas and H2O through well-known catalytic reactions of hydrogen evolution and oxidation. This battery chemistry exhibits a discharge voltage of ~1.3 V, a rate capability of 100 mA cm−2 (36 s of discharge) and a lifetime of more than 10,000 cycles without decay. We achieve a gravimetric energy density of ~139 Wh kg−1 (volumetric energy density of ~210 Wh l−1), with the theoretical gravimetric energy density of ~174 Wh kg−1 (volumetric energy density of ~263 Wh l−1) in a 4 M MnSO4 electrolyte. The manganese–hydrogen battery involves low-cost abundant materials and has the potential to be scaled up for large-scale energy storage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Dunn, B., Kamath, H. & Tarascon, J. M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Rugolo, J. & Aziz, M. J. Electricity storage for intermittent renewable sources. Energy Environ. Sci. 5, 7151–7160 (2012).
Chen, H. S. et al. Progress in electrical energy storage system: a critical review. Prog. Nat. Sci. 19, 291–312 (2009).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Goodenough, J. B. & Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 22, 587–603 (2010).
Pavlov, D. Lead–Acid Batteries: Science and Technology: A Handbook of Lead–Acid Battery Technology and its Influence on the Product (Elsevier, Amsterdam, 2011).
Wang, W. et al. Recent progress in redox flow battery research and development. Adv. Func. Mater. 23, 970–986 (2013).
Noack, J., Roznyatovskaya, N., Herr, T. & Fischer, P. The chemistry of redox-flow batteries. Angew. Chem. Int. Ed. 54, 9775–9808 (2015).
Huskinson, B. et al. A metal-free organic–inorganic aqueous flow battery. Nature 505, 195–198 (2014).
Yang, Y., Zheng, G. Y. & Cui, Y. A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy Environ. Sci. 6, 1552–1558 (2013).
Oshima, T., Kajita, M. & Okuno, A. Development of sodium–sulfur batteries. J. Appl. Ceram. Tech. 1, 269–276 (2004).
Wang, K. L. et al. Lithium–antimony–lead liquid metal battery for grid-level energy storage. Nature 514, 348–350 (2014).
Kim, H. et al. Liquid metal batteries: past, present, and future. Chem. Rev. 113, 2075–2099 (2013).
Ovshinsky, S. R., Fetcenko, M. A. & Ross, J. A nickel metal hydride battery for electric vehicles. Science 260, 176–181 (1993).
Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115–1118 (1994).
Beck, F. & Ruetschi, P. Rechargeable batteries with aqueous electrolytes. Electrochim. Acta. 45, 2467–2482 (2000).
Zhang, K. et al. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 44, 699–728 (2015).
Wei, W. F., Cui, X. W., Chen, W. X. & Ivey, D. G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 40, 1697–1721 (2011).
Pan, H. L. et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016).
Cottrell, F. G. On the solubility of manganous sulphate. J. Phys. Chem. 4, 637–656 (1900).
Li, Y. G. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011).
Popczun, E. J. et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 9267–9270 (2013).
Izhar, S. & Nagai, M. Transition metal phosphide catalysts for hydrogen oxidation reaction. Catal. Today 146, 172–176 (2009).
Yang, X. G. & Wang, C. Y. Nanostructured tungsten carbide catalysts for polymer electrolyte fuel cells. Appl. Phys. Lett. 86, 224104 (2005).
Sheng, W. C., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 157, B1529–B1536 (2010).
Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255–2260 (2014).
Biswal, A., Tripathy, B. C., Sanjay, K., Subbaiah, T. & Minakshi, M. Electrolytic manganese dioxide (EMD): a perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv. 5, 58255–58283 (2015).
Toupin, M., Brousse, T. & Belanger, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 16, 3184–3190 (2004).
Sun, M. et al. Controlled synthesis of nanostructured manganese oxide: crystalline evolution and catalytic activities. Cryst. Eng. Comm. 15, 7010–7018 (2013).
Electric Energy Storage Technology Options: A White Paper Primer on Applications, Costs, and Benefits Electric Power Research Institute Technical Report 1020676 (Electric Power Research Institute, 2010).
Roberts, B. P. & Sandberg, C. The role of energy storage in development of smart grids. Proc. IEEE 99, 1139–1144 (2011).
Acknowledgements
This work was initiated by the support of the Department of Energy, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, under contract DE-AC02-76-SFO0515.
Author information
Authors and Affiliations
Contributions
W.C. and Y.C. conceived the idea. W.C. designed the battery cells and conducted the electrochemical measurements. W.C. conducted SEM and XRD characterization. A.P. performed the simulation. Y.L. conducted TEM characterization. H.W. performed the XPS analysis. G.C. helped with the GC measurements. Y.C. supervised the project. W.C. and Y.C. contributed to writing the manuscript. W.C. and G.L. contributed equally to this work. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–44, Supplementary Notes 1–8, Supplementary Tables 1–2, Supplementary References
Rights and permissions
About this article
Cite this article
Chen, W., Li, G., Pei, A. et al. A manganese–hydrogen battery with potential for grid-scale energy storage. Nat Energy 3, 428–435 (2018). https://doi.org/10.1038/s41560-018-0147-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-018-0147-7
This article is cited by
-
Reversible multielectron transfer I−/IO3− cathode enabled by a hetero-halogen electrolyte for high-energy-density aqueous batteries
Nature Energy (2024)
-
Best practices for zinc metal batteries
Nature Sustainability (2024)
-
Discovering Cathodic Biocompatibility for Aqueous Zn–MnO2 Battery: An Integrating Biomass Carbon Strategy
Nano-Micro Letters (2024)
-
A recyclable biomass electrolyte towards green zinc-ion batteries
Nature Communications (2023)
-
Discovery of a three-proton insertion mechanism in α-molybdenum trioxide leading to enhanced charge storage capacity
Nature Communications (2023)