Abstract
Wildlife must adapt to human presence to survive in the Anthropocene, so it is critical to understand species responses to humans in different contexts. We used camera trapping as a lens to view mammal responses to changes in human activity during the COVID-19 pandemic. Across 163 species sampled in 102 projects around the world, changes in the amount and timing of animal activity varied widely. Under higher human activity, mammals were less active in undeveloped areas but unexpectedly more active in developed areas while exhibiting greater nocturnality. Carnivores were most sensitive, showing the strongest decreases in activity and greatest increases in nocturnality. Wildlife managers must consider how habituation and uneven sensitivity across species may cause fundamental differences in human–wildlife interactions along gradients of human influence.
Similar content being viewed by others
Main
With the global human population size now past 8 billion and the associated human footprint covering much of the Earth’s surface1, survival of wild animals in the Anthropocene requires that they adapt to physical changes to the landscape and to increasing human presence. Animals often perceive humans as threats and subsequently adjust behaviours to avoid people in space or time2. Conversely, some animals are attracted to people to obtain resource subsidies or protection from predators3,4. These contrasting responses to humans shape the prospects for human–wildlife coexistence, with consequences for the capacity of human-influenced ecosystems to support robust animal populations and communities.
Variation in animal responses to human activity can be driven by intrinsic factors such as species’ ecological and life-history traits (Table 1)5. For instance, small-bodied generalist species may be more tolerant of human presence, as they can be less conspicuous than larger species and more capable of shifting resource use within their broader niches than are specialists6. Wide-ranging, large-bodied carnivores face considerable risk of mortality from humans7 and so may exhibit more risk-averse responses to human activity. Animal responses may also be heavily influenced by the type of human activity (for example, hunting versus hiking8) and by extrinsic factors such as landscape context. Animals may be warier of people in open or human-modified environments relative to areas with abundant vegetation cover or minimal human landscape modification9. Conversely, animals in heavily modified landscapes could habituate to human presence and thus be less likely to respond to changes in human activity. Our ability to resolve such hypotheses about the interacting influences of species traits and landscape characteristics has been limited by the focus of previous studies on few species and contexts, with indirect measures of human activity and weaker correlative inferences. Ultimately, anticipating and managing impacts to wild animals requires stronger inferences from experimental manipulations of human activity and concurrent monitoring of people and animals across a range of species and environmental contexts.
Government policies during the early months of the COVID-19 pandemic (henceforth, pandemic) resulted in widespread changes to human activity that provided a quasi-experimental opportunity to study short-term behavioural responses of wild animals10. Early observations of animal responses to this ‘anthropause’11 relied on qualitative or opportunistic sightings prone to bias (for example, contributed by volunteers12), or focused on small spatial scales and few species, reporting a mix of positive and negative responses that make it difficult to reach more general conclusions13. Furthermore, measures of human activity have typically been coarse and indirect14, yet changes to human activity during the pandemic appeared highly variable at the fine scales that affect animal behaviour (Fig. 1). For example, some natural areas experienced increases in human visitation while others were closed to visitors15 and the strength of government restrictions changed over time14. It is thus important for studies using the pandemic as an unplanned experiment to have localized information on human activity that matches their animal data and to tackle context-dependency by using robust, standardized methods across several species and landscapes.
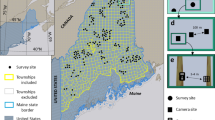
a, Location of camera-trap projects included in the analysis (n = 102). b,c, Examples for two projects: Edmonton, Canada (b) and Danum Valley, Malaysia (c) showing time series of human detections for the two types of comparisons used to assess the effects of higher human activity on animals. b, A between-year comparison with increased human activity during the COVID-19 pandemic (treatment, red shading) relative to the same time period the year before (control, blue shading). c, A within-year comparison with decreased human activity during the pandemic (control, blue shading) relative to the prepandemic period (treatment, red shading).
The widespread use of camera traps to survey terrestrial mammals16 provides a unique opportunity to take advantage of the pandemic experiment and improve our understanding of animal responses to changes in human activity. Thousands of cameras are deployed around the world17, providing standardized animal sampling while simultaneously quantifying local human activity15,18. We harnessed this opportunity to examine relationships between detections of people and mammals across gradients in land use and habitat type—spanning 102 survey sites (projects) in 21 countries (predominantly in Europe and North America) with 5,400 camera-trap locations sampling for 311,208 camera-days before and during the pandemic (Fig. 1; Methods). Some sites experienced a decrease in human activity during the pandemic, consistent with the notion of an anthropause, while there was an increase or no change at others. We focused our analysis on those sites with some change in human activity (either increase or decrease) and standardized our comparisons to be between periods of relatively lower to higher human activity (either across years or within 2020; Fig. 1; Methods) to mimic the general trend of increasing human presence in the Anthropocene. We examined site-level changes in animal detection rates and nocturnality across populations of 163 mammal species (body mass ≥ 1 kg; range 1–65 populations per species; Supplementary Table 1) as measures of the relative amount and timing of animal activity (Methods). We then used meta-analytic mixed-effects models to quantify the extent to which variation in animal responses across sites was explained by species traits, landscape modification and other site characteristics and the magnitude of change in human activity (Table 1; Methods).
Results and discussion
Our camera-trap measures of human activity varied widely under COVID-19 lockdowns (occurring between March 2020 and January 2021), from 100-fold decreases to 10-fold increases within sites between comparison periods (Fig. 1 and Supplementary Fig. 1). These changes were not predicted by coarser measures of human activity based on the stringency of lockdowns (Supplementary Fig. 1), highlighting the complementary value of finer-scaled monitoring of human activity.
Changes in amount of animal activity
Animals did not show consistent, negative responses to greater human activity; instead, responses were highly variable among species and sites (Figs. 2 and 3). Across 1,065 estimated responses (one per species per project, that is, population), changes in animal detection rates (reflecting the intensity of habitat use; Methods) varied from 139-fold increases to 36-fold decreases, with a near-zero mean change overall (−0.04, 95% confidence interval (CI) = −0.11–0.03; Fig. 2b). Trophic group (combining body mass and trophic level) was the strongest predictor of changes in animal activity in response to increasing human use, with large herbivores showing the largest increases in activity and carnivores showing the strongest decreases (Fig. 2c, Supplementary Table 2 and Supplementary Fig. 3). This is consistent with carnivore avoidance of higher mortality risk from encounters with people7 and with increased herbivore activity due to either more frequent disturbance by people or attraction to human activity driven by reduced risk of predation (human shield hypothesis3).
a, Interpretation of effects. b, Estimated effect sizes (black points) and variances (coloured lines) for all populations included in the analysis (n = 1,065 project–species combinations from 102 independent projects; two example species highlighted) with the global mean (and 95% quantiles) plotted in black to the right. c, Estimated model coefficients (points) and 95% CIs (lines; n = 1,065 project–species combinations from 102 independent projects) for additive factors (with complete data; Methods) hypothesized to influence changes in the amount of animal activity when human activity is higher, where: intercept is diurnal, large herbivore in closed habitat type with a seasonal comparison and all other effects are contrasts. d, Model predictions for the interaction between trophic group and HMI.
a, Interpretation of effects. b, Estimated effect sizes (black points) and variances (coloured lines) for all populations included in the analysis (n = 499 project–species combinations from 100 independent projects; two example species highlighted) with the global mean (with 95% quantiles) plotted in black to the right. c, Estimated model coefficients (points) and 95% CIs (lines; n = 499 project–species combinations from 100 independent projects) for additive factors (with complete data; Methods) hypothesized to influence changes in animal nocturnality when human activity is higher, where: intercept is nocturnal, large herbivore in closed habitat type with a seasonal comparison and all other effects are contrasts. d, Model predictions for interaction between trophic group and human modification index. e, Model predictions for interaction between hunting and HMI.
Animal activity in more developed areas (that is, higher human modification index (HMI) measured at the site level; Table 1) generally increased (+25%) with higher levels of human activity, while animals in less-developed areas decreased their activity (−6%) when human activity was higher (Fig. 2c; coefficient = 0.077; 95% CI = −0.001–0.156). This contrast highlights an important interaction between human modification of a landscape and human activity therein—between human footprint and footfalls—which we posit could be the result of two factors. First, local extirpations of sensitive species (species ‘filtering’19) would result in only human-tolerant species persisting in developed areas—for example, sensitive wolverine (Gulo gulo) were absent from sites with intermediate to high human modification. Second, species found across the gradient, such as mule deer (Odocoileus hemionus), could become habituated to benign human presence in more developed landscapes and therefore be less fearful of human activity than their conspecifics in less-developed areas20. Notably, this relationship with landscape modification varied predictably across trophic groups (Fig. 2d and Supplementary Table 3). Small and large carnivores, small herbivores and small omnivores increased their activity with higher human activity in developed areas (increasing by an average of 54%), while the response was much weaker for large herbivores and in fact opposite for large omnivores, which decreased activity when human activity increased in more modified landscapes (50% decrease; Fig. 2d). This negative response was common across all of the frequently detected large omnivores—wild boar (Sus scrofa), American black bear (Ursus americanus) and brown bear (Ursus arctos)—and could be driven by their attraction to anthropogenic food resources (for example garbage and fruit trees) that may be less risky to access when human activity is reduced21.
Animal detections were also more likely to decline with higher human activity in more open habitat types such as grasslands or deserts, relative to closed habitats such as forests (Fig. 2c; coefficient = −0.172; 95% CI = −0.3428 to −0.0018). This is consistent with predictions under the landscape of fear framework that suggest that animal perceptions of risk are influenced by availability of cover22. Contrary to our expectations, we did not find strong evidence that the magnitude of change in human activity (measured by camera traps or the stringency index; Table 1) affected animal responses or that hunted populations changed their amount of activity more than non-hunted ones (Supplementary Tables 2, 4 and 5). We also did not find strong support for the hypothesis that species with relatively larger brains—as an index of behavioural plasticity23—would show more pronounced responses to changes in human activity (Supplementary Table 5).
Changes in timing of animal activity
Whether or not animals change their intensity of use of an area, they could shift their timing of activity to minimize overlap with increasing human activity (Fig. 3a)24. We measured changes in animal nocturnality (proportion of night time detections) across 499 populations (Methods) and found considerable variation in animal responses to increasing human activity (though generally less than for amount of activity): from fivefold increases in nocturnality to sixfold decreases (mean change in proportion of nocturnal detections = 0.008; 95% CI = −0.02–0.04; Fig. 3b). The strongest predictor of changes in nocturnality was the degree of landscape modification (HMI): in more developed areas, animals tended to become more nocturnal as human activity increased (19.3% increase in nocturnality; Fig. 3c, coefficient = 0.047; 95% CI = 0.026–0.069; Supplementary Table 6). This is consistent with previous evidence of increasing wildlife nocturnality in the face of growing human impacts24 and highlights the importance of the temporal refuge provided by night time cover for human–wildlife coexistence in increasingly human-dominated environments25.
Paralleling our findings about changes in the amount of animal activity, trophic group was also an important predictor of changes in nocturnality, with large carnivores becoming notably more nocturnal than other groups (+5.3%; Fig. 3c and Supplementary Table 6). Again, we found support for an interaction between human modification and trophic group: most groups had stronger increases in nocturnality along the disturbance gradient as human activity increased (mean +22.6%), whereas the increases in nocturnality for large carnivores did not vary with land-use disturbance (Fig. 3d and Supplementary Table 7). This finding could reflect greater sensitivity of large carnivores to the increased risk of conflict associated with more human presence26, such that they shift timing of activity to minimize overlap regardless of landscape context. Other groups increased night time activity only in landscapes with higher risk of human encounters (that is, more modification), which may in turn enable the increases in amount of activity observed for many of these species (Fig. 2d).
Unlike for the amount of activity, changes in the timing of animal activity were mediated by the hunting status of species in an area, whereby hunted animals showed stronger increases in nocturnal behaviour at higher levels of landscape modification (+26.6%) relative to their non-hunted counterparts (+13.5%; Fig. 3e and Supplementary Table 8). We did not find strong evidence that relative brain size was associated with shifts in animal nocturnality, nor that the magnitude of change in the amount of human activity explained variation in animal responses (Fig. 3c and Supplementary Tables 6 and 9). We did find an effect of our comparison type such that, on average, comparisons between years showed larger shifts in nocturnality than within-year comparisons (Fig. 3c and Supplementary Table 6), underscoring the importance of temporal matching to minimize influence of other factors such as seasonal changes in activity patterns.
Implications for human–wildlife coexistence
Contrary to popular narratives of animals roaming more widely while people sheltered in place during early stages of the COVID-19 pandemic, our results reveal tremendous variation and complexity in animal responses to dynamic changes in human activity. Using a unique synthesis of simultaneous camera-trap sampling of people and hundreds of mammal species around the world, combined with a powerful before–after quasi-experimental design, we quantified how animals change their behaviours under higher levels of human activity across gradients of human footprint. As the human population continues to grow, the persistence of wild animals will depend on their responses to increasing human presence in both highly and moderately modified landscapes. It may thus be encouraging that many animal populations did not show dramatic changes in the amount or timing of their activity under conditions of higher human activity. Indeed, mean changes across all populations assessed were close to zero, suggesting that there was no global systematic shift in animal activity during the pandemic, consistent with other recent observations of highly variable animal responses13,27. Nevertheless, we saw stronger responses to human activity for certain species and contexts and these patterns can help us better understand and mitigate negative impacts of people on wildlife communities.
One striking pattern is that animal responses to human activity varied with the degree of human landscape modification. Our results imply that risk tolerance and associated behaviours vary between wildlife in more- versus less-developed contexts. As human activity increased, many species in more modified landscapes surprisingly had higher overall activity, although this activity was more nocturnal, suggesting that animals persisting in these developed environments may be attracted to anthropogenic resource subsidies but still seek ways to minimize encounters with people through partitioning time28. Wildlife managers in such modified environments should anticipate some animal habituation and manage the timing of human activity to protect night time refuges that promote human–wildlife coexistence—particularly for hunted species that showed the strongest shifts toward nocturnality. On the other hand, regulating the amount of human activity may be more important in less-developed landscapes where we detected the greatest declines in animal activity with increasing human activity. Such remote landscapes are often spatial refuges for sensitive species that may be filtered out as human modification increases; yet these areas face increasing demands from popular pursuits, such as outdoor recreation and nature-based tourism18, and may also be more difficult to protect from illegal hunting, encroachment or resource extraction29.
The sensitivity of species to human footprint and footfalls varied by trophic group and body size, as did the interplay of space and time in behavioural responses. Both large and small carnivore species were among the more sensitive to changes in human activity, generally reducing their activity levels and exhibiting more nocturnality with higher human activity. This motivates a continued emphasis on carnivore behaviour and management as a key challenge for human–wildlife coexistence, given the threatened status of many carnivores, the risk of negative outcomes of human–carnivore encounters and the ecological importance of carnivores as strongly interacting species7,30. Avoidance of people by carnivores could be beneficial if it reduces human–carnivore conflict25,28 but it could also lead to different types of conflict if it results in lower predation rates on herbivores near people, as seen in overbrowsing by habituated deer4. Indeed, large herbivores showed the strongest increases in activity with higher human activity in our study, consistent with habituation and increased risk of conflict. Large omnivores, such as bear and boar, were unique in both spatially and temporally avoiding higher human activity in more developed environments, underscoring that management efforts to regulate human activity and create spatial or temporal refuges may lead to outcomes that differ by species and setting. Managers must pay particular attention to the prospect that such differential responses can alter species interactions and cause knock-on effects with broader consequences for ecosystem functions and services31,32.
Our study highlights the value of learning from unplanned ‘experiments’ caused by rapid changes in human activity33 and other extreme events (for example, ref. 34). These insights are enabled by sampling methods, such as camera trapping, that facilitate standardized, continuous monitoring of diverse animal assemblages and humans across varied landscape contexts. While many studies of the anthropause focused on wildlife observations by volunteers in more accessible urban environments (for example, ref. 35), our results emphasize that animal responses to changes in human activity differ between more- and less-developed landscapes. This context-dependency should be a focus of further research, including expanded assessment of contexts and species under-represented in our sample, such as those in tropical regions subjected to different pressures during the pandemic36. Many geographic and taxonomic gaps in global biodiversity monitoring remain and must be filled by cost-effective networks that gather reliable evidence across several scales; standardized camera-trap programmes and infrastructure are helping to do so37,38. As the cumulative effects of the human enterprise put pressure on ecosystems worldwide39, bending the curve of biodiversity loss will require context-specific knowledge on ecological responses to human actions that can guide locally appropriate and globally effective conservation solutions.
Methods
Data collection
We issued a call in September 2020 to camera-trap researchers around the world for contributions of camera-trap data from before and during the onset of the COVID-19 pandemic and associated restrictions on human activity10,11. This initial call included a social media post (Twitter, now X) and targeted emails to 143 researchers in 37 countries. We requested datasets that adhered to global camera-trap metadata standards (Wildlife Insights38) and received submissions from 146 projects. Submitted data were summarized using a standardized script and evaluated according to the following key criteria: (1) most or all camera-trap stations were deployed in the same area of interest (hereafter site) before and during COVID-19-related restrictions; (2) a minimum of seven unique camera-trap deployment locations (stations) were sampled; (3) a minimum sampling effort of at least 7 days per camera period (see below); and (4) trends in human detections were recorded from camera-trap data (that is, detections of humans) or human activity for a given sampling area was available from other sources (for example, lockdown dates and local knowledge).
We only included detections of wild mammal species ≥1 kg (mean species body mass in kg obtained from ref. 40; we excluded domestic animals, which represented only 6% of overall detections and were associated with humans) and humans (excluding research personnel servicing cameras). Our full dataset for the next step of analysis included 112 projects sampling across 5,653 cameras for 329,535 camera-days (see below for data included in specific models). The mean number of camera locations per project was 42 (range 6–300) and mean camera-days per project was 2,945 (range 348–27,986). Camera locations were considered independent within projects, as no paired cameras were included (see Supplementary Table 10 for more details on camera deployments and spacing).
Experimental design
For each project, we first reviewed site-level trends in independent detection events of humans (using a standardized 30 min interval: that is, a detection was considered independent if >30 min from previous detection at the same camera station) to identify whether there were changes in human activity associated with COVID-19 restrictions in 2020. We sought to identify two comparable sampling periods that differed in human activity but were otherwise similar (for example, in camera locations and sampling effort) and thus could be used as a quasi-experimental comparison to assess wildlife responses to the change in human activity. We initially anticipated that human activity would be reduced during COVID-19 lockdowns (that is, the anthropause11) but observed a wide variety of patterns of human detections across datasets, including decreases, increases and no change in human detections between sampling before and during COVID-19 (Supplementary Fig. 1). Since our primary interest was in evaluating wildlife responses to changes in human activity and in general we anticipate increases in human activity during the Anthropocene, we standardized our treatments to represent increases in human activity. In other words, we defined a ‘control’ period as one with lower human activity and a ‘treatment’ period as one with higher human activity, regardless of which occurred before or during the COVID-19 pandemic (Fig. 1).
We identified start and end dates for each period on the basis of clear changes in human detections (determined from visual inspection of daily detections; Fig. 1). For some projects, dates corresponded to known dates of local COVID-19 lockdowns or changes in study design (for example, dates of camera placement or removal). We prioritized comparison between years when data were collected in similar periods in years before 2020 (n = 95 projects). If multiyear data were not available, we selected comparison periods before and after the onset of lockdowns around March 2020 (with specific dates chosen according to local lockdown conditions; n = 17). If there were several potential treatment periods, we prioritized periods on the basis of the following ordered criteria: (1) the fewest seasonal or ecological confounds; (2) the most similar study design; (3) the greatest sampling effort; and (4) the most recent time period. Of the 95 projects for which we made comparisons between 2020 and a previous year, we used 2019 for 88 projects, 2018 for 6 and 2017 for 1.
In cases where there was no noticeable difference in human detections between candidate periods, or there were insufficient human detections from camera traps, we used other data or local knowledge of changes in human activity (for example, lockdown dates and visitor use data) from co-authors responsible for the particular project. Of the 112 projects included in our initial analyses, 15 used this expert opinion to determine changes in human activity. After completing our initial categorization of comparison periods, we shared details with all data contributors for review and adjustment, if necessary, based on expert knowledge of a given study area. Contributors were asked whether our delineation of sampling periods as being high versus low in human activity corresponded with their knowledge of the study system. We also asked them to consider whether other sources of environmental variation (for example, fire, drought, seasonal or interannual variation) or sampling design could confound the attribution of changes in wildlife detections to changes in human activity. After this evaluation and review, we retained 102 project datasets that had a detectable change in human activity between a treatment and control period for subsequent statistical modelling. These projects spanned 21 countries, mostly in North America and Europe but with some representation from South America, Africa and Southeast Asia (Fig. 1 and Supplementary Table 10).
Our paired treatment–control design makes several assumptions. For instance, we assumed that either: (1) changes in human activity occurred in the same direction throughout the entire study area within the treatment period; (2) the direction of the average effect was more important than variation in direction across camera sites; (3) variation in human activity within a study area was lower than differences in human activity between the treatment (higher activity) and control (lower activity) periods. By standardizing our treatment to be the period of higher human activity, we also assumed that the temporal direction of change did not affect animal responses.
Data analysis
We compared two response variables between treatment and control periods to assess wildlife responses to changes in human activity: the amount of animal activity and the timing of animal activity (described below). We used a two-stage approach in which we first estimated the direction and magnitude of change in these responses between periods for each species and then used a meta-analytical approach to evaluate the degree to which a set of candidate predictor variables explained variation in estimated responses. All data manipulation and analysis were done using R statistical software (v.4.1.3; ref. 41).
Amount of animal activity
To evaluate changes in the amount of animal activity, we quantified detection rates for each mammal species (and humans) at each camera for the treatment and control periods of each project. Specifically, we calculated the number of independent detections for a given species and camera station using a standardized 30 min interval (that is, detection was considered independent if >30 min from previous detection of the same species at the same camera station), while controlling for variation in sampling effort (log of camera-days included as an offset in models). We assumed that this detection rate (sometimes termed relative abundance index16) measured the relative intensity of habitat use by a species at a camera station, which reflects both the local abundance of the species (number of individuals in sampled area) and the movement patterns of individuals.
To quantify the magnitude of change in the amount of animal activity, we first ran single-species models to estimate changes in detection rates for species and humans between the comparison periods for each project. The response variable was the count of independent detection events, modelled as negative binomial, with an offset for active camera-days. Treatment was included as a fixed effect and a random intercept was included for camera station where the same camera locations were sampled in both periods (no random effect was included if a project used different camera locations between periods). All models were implemented using the glmmTMB package42. These models produced a regression coefficient (effect size) for each project–species population (humans and animals) representing the estimated magnitude of change in the amount of activity between the control period and the treatment period (and its corresponding sampling variance).
Timing of animal activity
To assess changes in timing of animal activity, we first classified each independent detection of a given species within a given project as ‘day’ or ‘night’. We used the lutz package to convert all local times to UTC43. We calculated the angle of the sun at the time of the first image in each detection using the sunAngle function in the oce package44, based on the UTC time and latitude and longitude of the camera deployment location. Negative sun angles corresponded to ‘night’ (between sunset and sunrise) and positive sun angles to ‘day’ (between sunrise and sunset). Following ref. 24, we calculated an index of nocturnality, N, as the proportion of independent camera-trap detections that occurred during the night (N = detections during night/ (detections during night + detections during day)) for all species which had ten or more detections in both the control and treatment periods. We then calculated the log risk ratio, RR and its corresponding sampling variance (weighted by sample size) between the treatment and control periods, pooled across all camera traps within a given study using the escalc() function within the metafor package45. This effect size compared the percentage of animal detections that occurred at night with high human activity (Nh) to night time animal activity under low human activity (Nl), with RR = ln(Nh/Nl)). A positive RR indicated a relatively greater degree of nocturnality in response to human activity, while a negative RR indicated reduced nocturnality.
Hypothesized explanatory variables
We identified and calculated a set of variables that we hypothesized would affect species responses to changes in human activity. These fell into four general classes: (1) species traits, (2) habitat (that is, vegetation) structure, (3) anthropogenic landscape modification and (4) magnitude of human change (Table 1). We did not include any covariates reflecting differences in camera-trap sampling protocols between projects, as our estimates of species responses were made within projects (that is, comparing treatment versus control periods) and thus sampling methods were internally consistent within projects (for example, camera placement and settings).
Species traits
We hypothesized that species with the following traits would be more sensitive to changes in human activity (that is, more vulnerable or risk averse): larger body mass46, higher trophic level46, narrower diet and habitat breadth47, diurnal activity46 and smaller relative brain size48. We extracted variables for each species from the COMBINE database40, the most comprehensive archive of several mammal traits curated to date (representing 6,234 species). Given that some traits in the database were imputed, we reviewed the designations for plausibility and cross-referenced the traits with other widely used databases—specifically Elton Traits49 and PanTHERIA50—and made the following corrections to the ‘activity cycle’ trait (diurnal, nocturnal and cathemeral): diurnal to cathemeral—Mellivora capensis, Neofelis nebulosa, Neofelis diardi; diurnal to nocturnal—Meles meles; nocturnal to diurnal—Phacochoerus africanus; nocturnal to cathemeral—Ursus americanus. To calculate relative brain size we divided log-transformed brain mass by log-transformed body mass (as in ref. 48). We combined body mass and trophic level into a new variable ‘trophic group’ (consisting of small- or large-bodied categories for each of the three trophic levels, Table 1). Dietary and habitat breadth are described in ref. 40.
We further hypothesized that animals in hunted populations would be more sensitive to changes in human activity. We requested that all data contributors complete a survey indicating whether a given species was hunted within their project survey area, from which we created a binary factor representing hunting status for each population (1 = hunted; 0 = not hunted).
Habitat structure
Camera-trap surveys included in our analysis covered an extensive range of biogeographic areas and habitat types. We made the simplifying assumption that species responses to changes in human activity would be most influenced by the degree of openness of habitat (that is, vegetation structure) in a sampling area. More specifically, we hypothesized that areas with more open habitat types would have higher visibility and thus less security cover for animals and thus that animals in these open habitats would be more sensitive to increases in human activity than would animals in more closed habitats with greater security cover51. We used the Copernicus Global Land Cover dataset (100 m resolution52) via Google Earth Engine to extract land cover class at each camera station. We then used the percentage canopy cover of the mode class across all cameras in a given project to define if the survey occurred in primarily closed (>70% canopy cover) or open habitat types (0–70% canopy cover).
Land cover disturbance
We posited that animal responses to changes in human activity would differ according to the degree of anthropogenic landscape modification (that is, human footprint1,53). More specifically, we identified two hypotheses that could underlie variation in species responses as a function of land cover disturbance. On the one hand, our ‘habituation hypothesis’ predicts that animals in more disturbed landscapes may be less sensitive to changes in human activity (relative to animals in undisturbed landscapes) and thus show less of a negative response or even a positive response as they have already behaviourally adapted to tolerate co-occurrence with people22. On the other hand, our ‘plasticity hypothesis’ predicts that the ability of animals to coexist with people in disturbed landscapes may be dependent on plasticity in animal behaviour22, such that animals in these landscapes may show more pronounced and rapid responses to changes in human activity (for example, avoidance of areas and times with greater chance of encountering people).
We initially characterized landscape disturbance using three variables accessed via Google Earth Engine: Gridded Population of the World (1 km resolution54), road density (m km−2, 8 km resolution; Global Roads Inventory Project55) and HMI (for 2016 at 1 km resolution), which represents a cumulative measure of the proportion of a landscape modified by 13 anthropogenic stressors53. Point values were extracted for each camera station in each site, then the project-level medians were used in analysis. As the median values of these three variables were highly correlated across projects (Supplementary Fig. 2), we only used HMI in our subsequent models.
Magnitude of human change
We expected that animal responses would be more pronounced in areas that underwent greater changes in human activity and we used two measures to assess the magnitude of those changes. At a coarse scale, we used the COVID-19 stringency index14, which characterizes the policies restricting human activities within a given geographic region at a daily time scale and has been widely used in studies of COVID-19 on human mobility and the environment (for example, ref. 13). We used the finest-scale regional data available for each project, which was usually at the country level, with the exception of three countries with province- or state-level data (Brazil, Canada and the United States). When projects spanned several countries, provinces or states, we used the stringency index for the region in which most cameras were located. For each region, we calculated the median stringency for the treatment and control sampling periods.
At a finer scale, we used the effect size for the modelled change in camera-trap detection rates of humans across all cameras in a project (as described above under ‘amount of animal activity’). Models with this variable excluded 15 projects that either did not detect humans with camera traps or the number of humans detected on cameras was not perceived by the data contributor to be an accurate reflection of change in human use for the sampled area.
Meta-analysis models
To understand which factors mediated the effect of increasing human use on animal activity, we ran mixed-effect meta-analytic models using the rma.mv() function of the metafor package45 on the effect sizes and sampling variances of the two response variables described above (amount and timing of animal activity). Our unit of observation for modelling was the estimated response for each project–species combination (that is, each animal population) and we included random intercepts for project and for species nested within family, to account for repeated observations within each of those higher-level groups and for phylogenetic relatedness within families. All continuous predictor variables (Table 1) were standardized to unit variance with a mean of zero using the stdize function in the MuMIn package56. We tested pairwise correlations among all predictor variables and found that none were highly correlated (that is, all below a threshold of Pearson | r| < 0.6; Supplementary Fig. 2) and thus all were retained for modelling.
We performed our analysis in three steps for each of the two wildlife response variables. First, we fit a global model including all hypothesized predictor variables for which we had complete data (excluding hunting status, relative brain size and empirical magnitude of human change, for which we had incomplete data and thus included in analysis of subsets of data, described below). Second, we used model selection to test for plausible interactions and nonlinear effects. Third, we used model selection on subsets of the full data to compare the global and interactions models with candidate models adding three more predictor variables with incomplete data.
Global model
As all of our predictor variables were independent, we used a global model approach that included additive fixed effects for all predictor variables (Table 1). We interpreted the P value of each effect contrast to indicate statistically significant support (at P < 0.05 or marginal support at P < 0.10) for a consistent effect direction of a given predictor and we used the estimated effect size as a measure of effect magnitude. We calculated the pseudo-R2 to estimate the total variation explained by our global models. We also calculated the I2 (ref. 57) of each global model to determine the amount of heterogeneity observed between the random effect levels; consistent variation in the response terms between projects, families and species would result in higher I2 values compared to the null model with no fixed effects. To aid interpretation, we present effect sizes in terms of the proportional change (%) in model-predicted responses across lowest-to-highest values for continuous predictors (for example, HMI) or between two categories of interest (for example, trophic groups).
Model selection of plausible interactions and nonlinear terms
To explore the possibility of context-specific effects of the predictors of wildlife responses to changes in human activity, we assessed a suite of ecologically plausible interaction and nonlinear (quadratic) terms through adding them in turn to the global model and using Akaike’s Information Criterion (corrected for small sample size, AICc) to find the most parsimonious model. We assessed the following terms: (1) ‘HMI * habitat_closure’, to evaluate the potential for habitat structure to mediate responses to human landscape modification; (2) ‘trophic_group * HMI’, to evaluate the potential for different trophic groups to respond to human modification in different ways; (3) ‘trophic_group * habitat_closure’, to evaluate the potential for different trophic groups to respond to habitat structure in different ways; and (4) HMI2, to assess nonlinear effects of wildlife responses to human modification. Models including the candidate interaction or nonlinear terms were compared to the global model without interaction terms using AICc (in the MuMIn package56) and were discussed above if they were within 2 AICc of the best-supported model and there was no simpler, nested model with more support.
Model selection on subsets of data
We had a small amount of missing information in the data available for assessing the effects of population hunting status, species relative brain size and empirical (that is, camera-trap-based) magnitude of change in human activity (91.7%, 98.8% and 86.5% of project–species had data for these variables, respectively). Therefore, we ran the same global model used for the full dataset on the subsetted data along with candidate models including each of these predictor variables and all plausible interactions of interest (as above). These additional candidate models were compared to the global model (run on the same partial dataset) using AICc and were discussed in the results if they resulted in a lower AICc value (that is, had more support than the global model, which was a simpler nested model).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used in this paper are available in Figshare, with the identifier: https://doi.org/10.6084/m9.figshare.23506536.
Code availability
The code used to analyse the data and create the figures in this paper are available in Figshare, with the identifier: https://figshare.com/articles/software/Analysis_R_Code/23506512.
References
Venter, O. et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016).
Suraci, J. P., Clinchy, M., Zanette, L. Y. & Wilmers, C. C. Fear of humans as apex predators has landscape‐scale impacts from mountain lions to mice. Ecol. Lett. 22, 1578–1586 (2019).
Berger, J. Fear, human shields and the redistribution of prey and predators in protected areas. Biol. Lett. 3, 620–623 (2007).
McShea, W. J. Ecology and management of white-tailed deer in a changing world. Ann. NY Acad. Sci. 1249, 45–56 (2012).
Suraci, J. P. et al. Disturbance type and species life history predict mammal responses to humans. Glob. Change Biol. 27, 3718–3731 (2021).
Pacifici, M. et al. Global correlates of range contractions and expansions in terrestrial mammals. Nat. Commun. 11, 2840 (2020).
Ripple, W. J. et al. Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014).
Kays, R. et al. Does hunting or hiking affect wildlife communities in protected areas? J. Appl. Ecol. 54, 242–252 (2017).
Reilly, C. M., Suraci, J. P., Smith, J. A., Wang, Y. & Wilmers, C. C. Mesopredators retain their fear of humans across a development gradient. Behav. Ecol. 33, 428–435 (2022).
Bates, A. E., Primack, R. B., Moraga, P. & Duarte, C. M. COVID-19 pandemic and associated lockdown as a ‘Global Human Confinement Experiment’ to investigate biodiversity conservation. Biol. Conserv. 248, 108665 (2020).
Rutz, C. et al. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 4, 1156–1159 (2020).
Basile, M., Russo, L. F., Russo, V. G., Senese, A. & Bernardo, N. Birds seen and not seen during the COVID-19 pandemic: the impact of lockdown measures on citizen science bird observations. Biol. Conserv. 256, 109079 (2021).
Bates, A. E. et al. Global COVID-19 lockdown highlights humans as both threats and custodians of the environment. Biol. Conserv. 263, 109175 (2021).
Hale, T. et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 5, 529–538 (2021).
Procko, M., Naidoo, R., LeMay, V. & Burton, A. C. Human impacts on mammals in and around a protected area before, during and after COVID-19 lockdowns. Conserv. Sci. Pract. 4, e12743 (2022).
Burton, A. C. et al. Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 52, 675–685 (2015).
Chen, C. et al. Global camera trap synthesis highlights the importance of protected areas in maintaining mammal diversity. Conserv. Lett. 15, e12865 (2022).
Naidoo, R. & Burton, A. C. Relative effects of recreational activities on a temperate terrestrial wildlife assemblage. Conserv. Sci. Pract. 2, e271 (2020).
Betts, M. G. et al. Extinction filters mediate the global effects of habitat fragmentation on animals. Science 366, 1236–1239 (2019).
Lowry, H., Lill, A. & Wong, B. B. M. Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549 (2013).
Klees van Bommel, J., Badry, M., Ford, A. T., Golumbia, T. & Burton, A. C. Predicting human–carnivore conflict at the urban–wildland interface. Glob. Ecol. Conserv. 24, e01322 (2020).
Gaynor, K. M., Brown, J. S., Middleton, A. D., Power, M. E. & Brashares, J. S. Landscapes of fear: spatial patterns of risk perception and response. Trends Ecol. Evol. 34, 355–368 (2019).
González-Lagos, C., Sol, D. & Reader, S. M. Large-brained mammals live longer. J. Evol. Biol. 23, 1064–1074 (2010).
Gaynor, K. M., Hojnowski, C. E., Carter, N. H. & Brashares, J. S. The influence of human disturbance on wildlife nocturnality. Science 360, 1232 (2018).
Carter, N. H., Shrestha, B. K., Karki, J. B., Pradhan, N. M. B. & Liu, J. Coexistence between wildlife and humans at fine spatial scales. Proc. Natl Acad. Sci. USA 109, 15360–15365 (2012).
Packer, C. et al. Conserving large carnivores: dollars and fence. Ecol. Lett. 16, 635–641 (2013).
Tucker, M. A. et al. Behavioral responses of terrestrial mammals to COVID-19 lockdowns. Science 380, 1059–1064 (2023).
Lamb, C. T. et al. The ecology of human–carnivore coexistence. Proc. Natl Acad. Sci. USA 117, 17876 (2020).
Ripple, W. J. et al., Bushmeat hunting and extinction risk to the world’s mammals. R. Soc. Open Sci. 3, 160498 (2016).
Soulé, M. E., Estes, J. A., Berger, J. & Del Rio, C. M. Ecological effectiveness: conservation goals for interactive species. Conserv. Biol. 17, 1238–1250 (2003).
Estes, J. A. et al. Trophic downgrading of planet earth. Science 333, 301–306 (2011).
Raynor, J. L., Grainger, C. A. & Parker, D. P. Wolves make roadways safer, generating large economic returns to predator conservation. Proc. Natl Acad. Sci. USA 118, e2023251118 (2021).
Rutz, C. Studying pauses and pulses in human mobility and their environmental impacts. Nat. Rev. Earth Environ. 3, 157–159 (2022).
Ward, M. et al. Impact of 2019–2020 mega-fires on Australian fauna habitat. Nat. Ecol. Evol. 4, 1321–1326 (2020).
Schrimpf, M. B. et al. Reduced human activity during COVID-19 alters avian land use across North America. Sci. Adv. 7, eabf5073 (2021).
Lindsey, P. et al. Conserving Africa’s wildlife and wildlands through the COVID-19 crisis and beyond. Nat. Ecol. Evol. 4, 1300–1310 (2020).
Kays, R. et al. SNAPSHOT USA 2020: a second coordinated national camera trap survey of the United States during the COVID-19 pandemic. Ecology 103, e3775 (2022).
Ahumada, J. A. et al. Wildlife Insights: a platform to maximize the potential of camera trap and other passive sensor wildlife data for the planet. Environ. Conserv. 47, 1–6 (2019).
Díaz, S. et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366, eaax3100 (2019).
Soria, C. D., Pacifici, M., Di Marco, M., Stephen, S. M. & Rondinini, C. COMBINE: a coalesced mammal database of intrinsic and extrinsic traits. Ecology 102, e03344 (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Teucher A., lutz: Look up time zones of point coordinates. R package version 0.3.1 (2019).
Kelley D., Richards C., oce: Analysis of oceanographic data. R package version 1.7-10 (2022).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Purvis, A., Gittleman, J. L., Cowlishaw, G. & Mace, G. M. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952 (2000).
Chichorro, F., Juslén, A. & Cardoso, P. A review of the relation between species traits and extinction risk. Biol. Conserv. 237, 220–229 (2019).
Benson-Amram, S., Dantzer, B., Stricker, G., Swanson, E. M. & Holekamp, K. E. Brain size predicts problem-solving ability in mammalian carnivores. Proc. Natl Acad. Sci. USA 113, 2532–2537 (2016).
Wilman, H. et al. EltonTraits 1.0: species-level foraging attributes of the world’s birds and mammals. Ecology 95, 2027 (2014).
Jones, K. E. et al. PanTHERIA: a species-level database of life history, ecology and geography of extant and recently extinct mammals. Ecology 90, 2648 (2009).
Stankowich, T. Ungulate flight responses to human disturbance: a review and meta-analysis. Biol. Conserv. 141, 2159–2173 (2008).
Buchhorn, M. et al. Copernicus global land cover layers—Collection 2. Remote Sens. 12, 1044 (2020).
Kennedy, C. M., Oakleaf, J. R., Theobald, D. M., Baruch-Mordo, S. & Kiesecker, J. Managing the middle: a shift in conservation priorities based on the global human modification gradient. Glob. Change Biol. 25, 811–826 (2019).
Gridded Population of the World, Version 4 (GPWv4): Population Density (CIESIN, 2016).
Meijer, J. R., Huijbregts, M. A., Schotten, K. C. & Schipper, A. M. Global patterns of current and future road infrastructure. Environ. Res. Lett. 13, 064006 (2018).
Bartoń, K. MUMIn: Multi-model inference. R package version 1.47.1 (2022).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta‐analysis. Stat. Med. 21, 1539–1558 (2002).
Acknowledgements
We recognize the tragic consequences of the COVID-19 pandemic and would like to acknowledge all people impacted. Full acknowledgements are provided in the Supplementary Information. This synthesis project was funded by the Natural Sciences and Engineering Research Council of Canada (Canada Research Chair 950-231654 and Discovery Grant RGPIN-2018-03958 to A.C.B. and RGPIN-2022-03096 to K.M.G.) and the National Center for Ecological Analysis and Synthesis (Director’s Postdoc Fellowship to K.M.G.). Additional funding sources for component subprojects are listed in the Supplementary Information.
Author information
Authors and Affiliations
Contributions
A.C.B., C. Beirne, R.K., K.M.G., C. Sun and A. Granados conceived this work. A.C.B., C. Beirne, R.K., K.M.G., A. Granados, C. Sun and F.C. were responsible for data curation. C. Beirne and K.M.G. conducted the formal analysis. A.C.B., R.K. and K.M.G. acquired funding. A.C.B., C. Beirne, K.M.G., C. Sun, A. Granados, M.L.A., J.M.A., G.C.A., F.S.Á.C., Z.A., C.A.-D., C.A., S.A.-A., G.B., A.B.-M., D.B., E.B., E.L.B., C. Baruzzi, S.M.B., N. Beenaerts, J. Belmaker, O.B., B.B., T.B., D.A.B., N. Bogdanović, A.B., M.B., L.B., J.F.B., J. Brooke, J.W.B., F.C., B.S.C., J. Carvalho, J. Casaer, R. Černe, R. Chen, E.C., M.C., C. Cincotta, D.Ć., T.D.C., J. Compton, C. Coon, M.V.C., A.P.C., S.D.F., A.K.D., M. Davis, K.D., V.D.W., E.D., T.A.D., J.D., M. Duľa, S.E.-F., C.E., A.E., J.F.-L., J. Favreau, M.F., P.F., F.F., C.F., L.F., J.T.F., M.C.F.-R., E.A.F., U.F., J.F.l., J.M.F., A.F., B. Franzetti, S. Frey, S. Fritts, Š. Frýbová, B. Furnas, B.G., H.M.G., D.G.G., A.J.G., T.G., M.E.G., D.M.G., M.G., A. Green, R.H., R.(B.)H., S. Hammerich, C. Hanekom, C. Hansen, S. Hasstedt, M. Hebblewhite, M. Heurich, T.R.H., T.H., D.J., P.A.J., K.J.J., A.J., M.J., M.C.K., M.J.K., M.T.K., S.K.-S., M. Krofel, A.K., K.M.K., D.P.J.K., E.K.K., J.K., M. Kutal, D.J.R.L., S.L., M. Lashley, R. Lathrop, T.E.L.J., C.L., D.B.L., A.L., M. Linnell, J. Loch, R. Long, R.C.L., J. Louvrier, M.S.L., P.M., S.M., B.M., G.K.H.M., A.J.M., D.M., Z.M., T.M., W.J.M., M.M., C.M., J.J.M., C.M.M.-M., D.M.-A., K.M., C. Nagy, R.N., I.N., C. Nelson, B.O., M.T.O., V.O., C.O., F.O., P.P., K.P., L.P., C.E.P., M. Pendergast, F.F.P., R.P., X.P.-O., M. Price, M. Procko, M.D.P., E.E.R., N.R., S.R., K.R., M.R., R.R., R.R.-H., D.R., E.G.R., A.R., C. Rota, F.R., H.R., C. Rutz, M. Salvatori, D.S., C.M.S., J. Scherger, J. Schipper, D.G.S., Ç.H.Ş., P.S., J. Sevin, H.S., C. Shier, E.A.S.-R., M. Sindicic, L.K.S., A.S., T.S., C.C.S.C., J. Stenglein, P.A.S., K.M.S., M. Stevens, C. Stevenson, B.T., I.T., R.T.T., J.T., T.U., J.-P.V., D.V., S.L.W., J. Weber, K.C.B.W., L.S.W., C.A.W., J. Whittington, I.W., M.W., J. Williamson, C.C.W., T.W., H.U.W., Y.Z., A.Z. and R.K. carried out the investigations. A.C.B., R.K. and F.C. were responsible for project administration. A.C.B., C. Beirne, R.K., K.M.G., C. Sun and A. Granados wrote the original draft manuscript. A.C.B., C. Beirne, K.M.G., C. Sun, A. Granados, M. Lashley, J.M.A., G.C.A., F.S.Á.C., Z.A., C.A.-D., C.A., S.A.-A., G.B., A.B.-M., D.B., E.B., E.L.B., C. Baruzzi, S.M.B., N. Beenaerts, J. Belmaker, O.B., B.B., T.B., D.A.B., N. Bogdanović, A.B., M.B., L.B., J.F.B., J. Brooke, J.W.B., F.C., B.S.C., J. Carvalho, J. Casaer, R. Černe, R. Chen, E.C., M.C., C. Cincotta, D.Ć., T.D.C., J. Compton, C. Coon, M.V.C., A.P.C., S.D.F., A.K.D., M. Davis, K.D., V.D.W., E.D, T.A.D., J.D., M. Duľa, S.E.-F., C.E., A.E., J.F.-L., J. Favreau, M.F., P.F., F.F., C.F., L.F., J.T.F., M.C.F.-R., E.A.F., U.F., J.F.l., J.M.F., A.F., B. Franzetti, S. Frey, S. Fritts, Š. Frýbová, B. Furnas, B.G., H.M.G., D.G.G., A.J.G., T.G., M.E.G., D.M.G., M.G., A. Green, R.H., R.(B.)H., S. Hammerich, C. Hanekom, C. Hansen, S. Hasstedt, M. Hebblewhite, M. Heurich, T.R.H., T.H., D.J., P.A.J., K.J.J., A.J., M.J., M.C.K., M.J.K., M.T.K., S.K.-S., M. Krofel, A.K., K.M.K., D.P.J.K., E.K.K., J.K., M. Kutal, D.J.R.L., S.L., M. Lashley, R. Lathrop, T.E.L.J., C.L., D.B.L., A.L., M. Linnell, J. Loch, R. Long, R.C.L., J. Louvrier, M.S.L., P.M., S.M., B.M., G.K.H.M., A.J.M., D.M., Z.M., T.M., W.J.M., M.M., C.M., J.J.M., C.M.M.-M., D.M.-A., K.M., C. Nagy, R.N., I.N., C. Nelson, B.O., M.T.O., V.O., C.O., F.O., P.P., K.P., L.P., C.E.P., M. Pendergast, F.F.P., R.P., X.P.-O., M. Price, M. Procko, M.D.P., E.E.R., N.R., S.R., K.R., M.R., R.R., R.R.-H., D.R., E.G.R., A.R., C. Rota, F.R., H.R., C. Rutz, M. Salvatori, D.S., C.M.S., J. Scherger, J. Schipper, D.G.S., Ç.H.Ş., P.S., J. Sevin, H.S., C. Shier, E.A.S.-R., M. Sindicic, L.K.S., A.S., T.S., C.C.S.C., J. Stenglein, P.A.S., K.M.S., M. Stevens, C. Stevenson, B.T., I.T., R.T.T., J.T., T.U., J.-P.V., D.V., S.L.W., J. Weber, K.C.B.W., L.S.W., C.A.W., J. Whittington, I.W., M.W., J. Williamson, C.C.W., T.W., H.U.W., Y.Z., A.Z. and R.K. were involved in reviewing and editing the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Mason Fidino, Mahdieh Tourani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Extended Results, Supplementary Figs. 1–4, Tables 1–10 and full Acknowledgements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burton, A.C., Beirne, C., Gaynor, K.M. et al. Mammal responses to global changes in human activity vary by trophic group and landscape. Nat Ecol Evol (2024). https://doi.org/10.1038/s41559-024-02363-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-024-02363-2