Abstract
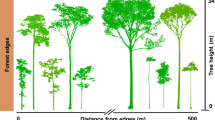
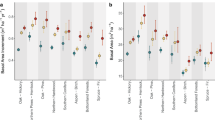
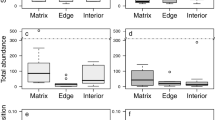
Forest biodiversity and ecosystem services are hitherto predominantly quantified in forest interiors, well away from edges. However, these edges also represent a substantial proportion of the global forest cover. Here we quantified plant biodiversity and ecosystem service indicators in 225 plots along forest edge-to-interior transects across Europe. We found strong trade-offs: phylogenetic diversity (evolutionary measure of biodiversity), proportion of forest specialists, decomposition and heatwave buffering increased towards the interior, whereas species richness, nectar production potential, stemwood biomass and tree regeneration decreased. These trade-offs were mainly driven by edge-to-interior structural differences. As fragmentation continues, recognizing the role of forest edges is crucial for integrating biodiversity and ecosystem service considerations into sustainable forest management and policy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data needed to reproduce the analyses and figures presented in this study are available on Figshare (https://doi.org/10.6084/m9.figshare.24559891.v3) and GitHub (https://github.com/to-vanneste/tradeoffs.git).
Code availability
All R code needed to reproduce the analyses and figures presented in this study is available on Figshare (https://doi.org/10.6084/m9.figshare.24559891.v3) and GitHub (https://github.com/to-vanneste/tradeoffs.git).
References
FAO. in FAO Global Forest Resource Assessment 2015 105 pp. (FAO, 2015).
Valdés, A. et al. High ecosystem service delivery potential of small woodlands in agricultural landscapes. J. Appl. Ecol. 57, 4–16 (2020).
Hertzog, L. R. et al. Forest fragmentation modulates effects of tree species richness and composition on ecosystem multifunctionality. Ecology 100, e02653 (2019).
Meeussen, C. et al. Microclimatic edge-to-interior gradients of European deciduous forests. Agric. Meteorol. 311, 108699 (2021).
Schmidt, M., Jochheim, H., Kersebaum, K. C., Lischeid, G. & Nendel, C. Gradients of microclimate, carbon and nitrogen in transition zones of fragmented landscapes—a review. Agric. Meteorol. 232, 659–671 (2017).
Remy, E., Wuyts, K., Boeckx, P., Gundersen, P. & Verheyen, K. Edge effects in temperate forests subjected to high nitrogen deposition. Proc. Natl Acad. Sci. USA 114, E7032 (2017).
Pfeifer, M. et al. Creation of forest edges has a global impact on forest vertebrates. Nature 551, 187–191 (2017).
Taubert, F. et al. Global patterns of tropical forest fragmentation. Nature 554, 519–522 (2018).
Haddad, N. M. et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 1, e1500052 (2015).
Riitters, K., Wickham, J., Costanza, J. K. & Vogt, P. A global evaluation of forest interior area dynamics using tree cover data from 2000 to 2012. Landsc. Ecol. 31, 137–148 (2016).
Estreguil, C., Caudullo, G., de Rigo, D. & San-Miguel-Ayanz, J. Forest Landscape in Europe: Pattern, Fragmentation and Connectivity EUR 25717 (Publications Office of the European Union, 2012).
Shapiro, A. C., Aguilar-Amuchastegui, N., Hostert, P. & Bastin, J. F. Using fragmentation to assess degradation of forest edges in Democratic Republic of Congo. Carbon Balance Manag. 11, 11 (2016).
Landuyt, D. et al. The functional role of temperate forest understorey vegetation in a changing world. Glob. Change Biol. 25, 3625–3641 (2019).
Thrippleton, T., Bugmann, H., Folini, M. & Snell, R. S. Overstorey–understorey interactions intensify after drought-induced forest die-off: long-term effects for forest structure and composition. Ecosystems 21, 723–739 (2018).
Chastain, R. A. Jr, Currie, W. S. & Townsend, P. A. Carbon sequestration and nutrient cycling implications of the evergreen understory layer in Appalachian forests. For. Ecol. Manag. 231, 63–77 (2006).
De Lombaerde, E. et al. Understorey removal effects on tree regeneration in temperate forests: a meta‐analysis. J. Appl. Ecol. 58, 9–20 (2021).
Perring, M. P. et al. Understanding context dependency in the response of forest understorey plant communities to nitrogen deposition. Environ. Pollut. 242, 1787–1799 (2018).
Zellweger, F. et al. Forest microclimate dynamics drive plant responses to warming. Science 368, 772–775 (2020).
Le Bagousse-Pinguet, Y. et al. Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl Acad. Sci. USA 116, 8419–8424 (2019).
van der Plas, F. et al. Jack-of-all-trades effects drive biodiversity–ecosystem multifunctionality relationships in European forests. Nat. Commun. 7, 11109 (2016).
Calders, K. et al. Nondestructive estimates of above‐ground biomass using terrestrial laser scanning. Methods Ecol. Evol. 6, 198–208 (2015).
Thompson, P. L. & Gonzalez, A. Ecosystem multifunctionality in metacommunities. Ecology 97, 2867–2879 (2016).
Gough, C. M., Atkins, J. W., Fahey, R. T. & Hardiman, B. S. High rates of primary production in structurally complex forests. Ecology 100, e02864 (2019).
Penone, C. et al. Specialisation and diversity of multiple trophic groups are promoted by different forest features. Ecol. Lett. 22, 170–180 (2019).
Ehbrecht, M. et al. Global patterns and climatic controls of forest structural complexity. Nat. Commun. 12, 519 (2021).
Karger, D. N., Nobis, M. P., Normand, S., Graham, C. H., & Zimmermann, N. E. CHELSA-TraCE21k v1. 0. Downscaled transient temperature and precipitation data since the Last Glacial Maximum. Clim. Past. 19, 439–456 (2023).
Brus, D. J. et al. Statistical mapping of tree species over Europe. Eur. J. For. Res. 131, 145–157 (2012).
Govaert, S. et al. Edge influence on understorey plant communities depends on forest management. J. Veg. Sci. 31, 281–292 (2020).
Meeussen, C. et al. Structural variation of forest edges across Europe. For. Ecol. Manag. 462, 117929 (2020).
De Pauw, K. et al. Taxonomic, phylogenetic and functional diversity of understorey plants respond differently to environmental conditions in European forest edges. J. Ecol. 109, 2629–2648 (2021).
Heinken, T. et al. The European Forest Plant Species List (EuForPlant): concept and applications. J. Veg. Sci. 33, e13132 (2022).
Zanne, A. E. et al. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014).
Laliberté, E. & Legendre, P. A distance‐based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010).
Meeussen, C. et al. Drivers of carbon stocks in forest edges across Europe. Sci. Total Environ. 759, 143497 (2021).
Tyler, T., Herbertsson, L., Olofsson, J. & Olsson, P. A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 120, 106923 (2021).
De Frenne, P. et al. Global buffering of temperatures under forest canopies. Nat. Ecol. Evol. 3, 744–749 (2019).
Zellweger, F. et al. Seasonal drivers of understorey temperature buffering in temperate deciduous forests across Europe. Glob. Ecol. Biogeogr. 28, 1774–1786 (2019).
Xu, S., Sardans, J., Zhang, J. & Peñuelas, J. Variations in foliar carbon: nitrogen and nitrogen: phosphorus ratios under global change: a meta-analysis of experimental field studies. Sci. Rep. 10, 12156 (2020).
Chen, X. & Chen, H. Y. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 12, 4562 (2021).
Muller, R. N. in The Herbaceous Layer in Forests of Eastern North America (ed. Gilliam, F.) Ch. 2 (Oxford University Press, 2014).
Mabry, C. M., Gerken, M. E. & Thompson, J. R. Seasonal storage of nutrients by perennial herbaceous species in undisturbed and disturbed deciduous hardwood forests. Appl. Veg. Sci. 11, 37–44 (2008).
Jenkins, J. C., Chojnacky, D. C., Heath, L. S. & Birdsey, R. A. National-scale biomass estimators for United States tree species. For. Sci. 49, 12–35 (2003).
Dzwonko, Z. & Gawroński, S. Effect of litter removal on species richness and acidification of a mixed oak-pine woodland. Biol. Conserv. 106, 389–398 (2002).
Verheyen, K. et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 100, 352–365 (2012).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Huey, R. B. et al. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. B 367, 1665–1679 (2012).
Hansen, M. C. et al. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013).
Beguería, S. and Vicente-Serrano, S. M. SPEI: calculation of the Standardised Precipitation-Evapotranspiration Index. R package version 1.7. R Project https://CRAN.R-project.org/package=SPEI (2017).
Harris, I., Osborn, T. J., Jones, P. & Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 7, 109 (2020).
Sousa‐Silva, R. et al. Tree diversity mitigates defoliation after a drought‐induced tipping point. Glob. Change Biol. 24, 4304–4315 (2018).
Grossiord, C. et al. Tree diversity does not always improve resistance of forest ecosystems to drought. Proc. Natl Acad. Sci. USA 111, 14812–14815 (2014).
Guada, G., Camarero, J. J., Sánchez-Salguero, R. & Cerrillo, R. M. N. Limited growth recovery after drought-induced forest dieback in very defoliated trees of two pine species. Front. Plant Sci. 7, 418 (2016).
Isbell, F. et al. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202 (2011).
Wuyts, K. et al. Comparison of forest edge effects on throughfall deposition in different forest types. Environ. Pollut. 156, 854–861 (2008).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Project https://www.R-project.org/ (2021).
Vanneste, T. Trade-offs in biodiversity and ecosystem services between edges and interiors in European forests. figshare https://doi.org/10.6084/m9.figshare.24559891.v3 (2023).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017).
Bürkner, P.-C. Bayesian item response modeling in R with brms and Stan. J. Stat. Softw. 100, 1–54 (2021).
Fox, J. & Weisberg, S. An R Companion to Applied Regression 3rd edn (Sage Publications, 2019).
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R Vol. 574 (Springer, 2009).
Gabry, J. & Mahr, T. bayesplot: plotting Bayesian models. R package version 1.8.1. bayesplot https://mc-stan.org/bayesplot/ (2021).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Slade, E. M. et al. The importance of species identity and interactions for multifunctionality depends on how ecosystem functions are valued. Ecology 98, 2626–2639 (2017).
Acknowledgements
Special thanks go to L. Willems and G. De Bruyn for performing the chemical analysis and to A. Ghrairi for the texture analysis. We also thank E. Ampoorter, H. Blondeel, F. Ceunen, K. Ceunen, R. De Beelde, E. De Lombaerde, K. Hansson, L. Hertzog, D. Landuyt, P. Lhoir, S. M. Krishna Moorthy, A. Peiffer, M. Perring, M. Tolosano, S. Van Den Berge, L. Van Nevel and M. Vedel-Sørensen for their assistance during the fieldwork. T.V., L.D., E.D.L., C.M., P.S., P.V. and P.D.F. received funding through the ERC Starting grant FORMICA (no. 757833, http://www.formica.ugent.be). S.G., K.D.P. and L.D. were supported by the Research Foundation Flanders (FWO) (nos. G0H1517N, ASP035-19 and 1221523N, respectively). The plot network and data collection were realized through the FWO Scientific research network FLEUR (http://www.fleur.ugent.be).
Author information
Authors and Affiliations
Contributions
T.V., L.D., P.D.F., P.V. and K.V. conceived and designed the study. E.D.L., C.M., S.G., K.D.P., P.S., K.B., J.B., K.C., S.A.O.C., M.D., C.G., B.J.G., P.-O.H., G.I., J.L., S.L., A.O., Q.P., J.P., F. Selvi, F. Spicher, H.V., F.Z. and P.V. collected the data. C.M., S.G., K.D.P. and P.S. processed the data, while T.V. and L.D. performed the data analyses under supervision of P.D.F. and K.V. T.V. and L.D. drafted the manuscript, and all authors contributed to later versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Jörg Albrecht and Xingfeng Si for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Study design and set-up.
Study design with broadleaved forests in nine regions spanning a ± 2300-km wide latitudinal gradient across the European sub-Mediterranean, temperate and boreonemoral forest biome. three forest stands were selected per region with contrasting management intensity: (1) ‘dense forests’ that where not thinned over the past 10–30 years, (2) ‘intermediate forests’ with frequent thinning and most recent thinning about 5–10 years ago, and (3) ‘open forests’ with regular thinning and most recent thinning less than 4 years before sampling. In each forest stand, a 100-m transect was established perpendicular to the south-facing forest edge. Five plots of 3 m × 3 m were installed along each transect, with their plot centres at an exponentially increasing distance from the focal forest edge (1.5, 4.5, 12.5, 35.5 and 99.5 m). All plots were at least 100 m away from any forest edge other than the focal forest edge. Figure adapted from Meeussen et al. (2021) with background map on the left from https://databasin.org/ and picture of the transect on the right from Google Earth (Map data ©Google 2020 Geobasis-DE/BKG ©2009, Google Imagery ©2020 TerraMetrics). Hemispherical pictures of the three different forest management types in the middle were taken during the fieldwork.
Extended Data Fig. 2 Schematic overview of the collected data and data analyses.
Selection of biodiversity and ecosystem service indices for which edge-to-interior patterns were investigated in deciduous forest stands across temperate Europe. Subsequently, the underlying effect of several environmental drivers acting across three biogeographical scales on these indicators was assessed to explain the observed edge-to-interior variation. Icons were extracted from The Noun Project (https://thenounproject.com).
Extended Data Fig. 3 Effect of design variables and environmental variables on an ecosystem multifunctionality index.
Effect of design variables, that is distance to the edge, latitude, forest density and elevation (A), and environmental drivers (B) on ecosystem multifunctionality quantified for each 3 m × 3 m plot in the forest-edge-to-interior transects (n = 225 biologically independent plots). To quantify ecosystem multifunctionality, we followed the desirability function approach, outlined in Slade et al.63. For each measured ecosystem function, we established a desirability function that describes how desirability changes in function of the measured value of the ecosystem function. For each ecological function yi, a desirability function assigns numbers between 0 and 1, with di = 0 representing a completely undesirable value of yi and di = 1 representing a completely desirable or ideal function value [3,63]. For each ecosystem function, we assumed a linear relationship with desirability, positive for all functions except summer offset, because a lower offset indicates more temperature buffering and is therefore more desirable during heatwave conditions. Each function was scaled relative to the minimum and maximum values in the dataset, that is for a positive relation, the lowest and highest observed value of the ecological function were given a desirability of 0 and 1, respectively. Next, we determined importance weights for each ecosystem function, and calculated an overall multifunctionality index as the weighted average of the desirability scores of all ecosystem functions. All functions were given an importance weight of 1, except for the five ecosystem function measures related to biodiversity (total richness, specialist richness, generalist richness, phylogenetic diversity, and functional diversity), whose weights were reduced to 0.2 to avoid overweighting of the biodiversity aspect in the final multifunctionality index. Circles represent mean standardized effect sizes with 80% (thick line) and 95% credible intervals (thin line) and distributions obtained from a Bayesian model.
Extended Data Fig. 4 Effect of edaphic properties and landscape-scale variables on biodiversity and ecosystem service indices.
Forest plots displaying the effect of soil texture (% sand), soil acidity (pH), litter quality (mass of the organic soil layer), habitat availability (forest cover in 500-m radius), drought (SPEI) and atmospheric pollution (N deposition) on the biodiversity and ecosystem service indices quantified for each 3 m × 3 m plot in the forest-edge-to-interior transects (n = 225 biologically independent plots). Circles represent mean standardized effect sizes with 80% (thick line) and 95 % credible intervals (thin line) and distributions obtained from a multivariate Bayesian model. Colours denote biodiversity indices (green), regulating (blue) and provisioning ecosystem services (orange).
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Tables 1–9 and Methods 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vanneste, T., Depauw, L., De Lombaerde, E. et al. Trade-offs in biodiversity and ecosystem services between edges and interiors in European forests. Nat Ecol Evol (2024). https://doi.org/10.1038/s41559-024-02335-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-024-02335-6