Abstract
Many organisms fail to adjust their phenology sufficiently to climate change. Studies have concentrated on adaptive responses within localities, but little is known about how latitudinal dispersal enhances evolutionary potential. Rapid adaptation is expected if dispersers from lower latitudes have improved synchrony to northern conditions, thereby gain fitness and introduce genotypes on which selection acts. Here we provide experimental evidence that dispersal in an avian migrant enables rapid evolutionary adaptation. We translocated Dutch female pied flycatchers (Ficedula hypoleuca) and eggs to Sweden, where breeding phenology is ~15 days later. Translocated females bred earlier, and their fitness was 2.5 times higher than local Swedish flycatchers. We show that between-population variation in timing traits is highly heritable, and hence immigration of southern genotypes promotes the necessary evolutionary response. We conclude that studies on adaptation to large-scale environmental change should not just focus on plasticity and evolution based on standing genetic variation but should also include phenotype–habitat matching through dispersal as a viable route to adjust.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data used in the analyses are attached in the Source Data and available from DataverseNL (https://doi.org/10.34894/TXG0GC). Source data are provided with this paper.
References
Walther, G. R. et al. Ecological responses to recent climate change. Nature 416, 389–395 (2002).
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003).
Root, T., Price, J., Hall, K. & Schneider, S. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (2003).
Thackeray, S. J. et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245 (2016).
Simmonds, E. G., Cole, E. F., Sheldon, B. C. & Coulson, T. Phenological asynchrony: a ticking time-bomb for seemingly stable populations? Ecol. Lett. 23, 1766–1775 (2020).
Both, C., Bouwhuis, S., Lessells, C. M. & Visser, M. E. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (2006).
Botero, C. A., Weissing, F. J., Wright, J. & Rubenstein, D. R. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189 (2015).
Charmantier, A. & Gienapp, P. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7, 15–28 (2014).
Edelaar, P. & Bolnick, D. I. Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665 (2012).
Nicolaus, M. & Edelaar, P. Comparing the consequences of natural selection, adaptive phenotypic plasticity, and matching habitat choice for phenotype–environment matching, population genetic structure, and reproductive isolation in meta-populations. Ecol. Evol. 8, 3815–3827 (2018).
Hušek, J., Lampe, H. M. & Slagsvold, T. Natal dispersal based on past and present environmental phenology in the pied flycatcher (Ficedula hypoleuca). Oecologia 174, 1139–1149 (2014).
Wilczek, A. M., Cooper, M. D., Korves, T. M. & Schmitt, J. Lagging adaptation to warming climate in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 111, 7906–7913 (2014).
Pelini, S. L. et al. Translocation experiments with butterflies reveal limits to enhancement of poleward populations under climate change. Proc. Natl Acad. Sci. USA 106, 11160–11165 (2009).
Schiffers, K., Bourne, E. C., Lavergne, S., Thuiller, W. & Travis, J. M. J. Limited evolutionary rescue of locally adapted populations facing climate change. Philos. Trans. R. Soc. B 368, 0–9 (2013).
Burger, C., Nord, A., Nilsson, J. Å., Gilot-Fromont, E. & Both, C. Fitness consequences of northward dispersal as possible adaptation to climate change, using experimental translocation of a migratory passerine. PLoS ONE 8, 1–9 (2013).
Sanz, J. J. Geographic variation in breeding parameters of the pied flycatcher Ficedula hypoleuca. Ibis 139, 107–119 (1997).
Both, C. et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B 271, 1657–1662 (2004).
Both, C. & Visser, M. E. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (2001).
Ouwehand, J. & Both, C. African departure rather than migration speed determines variation in spring arrival in pied flycatchers. J. Anim. Ecol. 86, 88–97 (2017).
Thomson, D. L., van Noordwijk, A. & Hagemeijer, W. Estimating avian dispersal distances from data on ringed birds. J. Appl. Stat. 30, 1003–1008 (2003).
Both, C., Robinson, R. A. & van der Jeugd, H. P. Long-distance dispersal in migratory pied flycatchers Ficedula hypoleuca is relatively common between the UK and the Netherlands. J. Avian Biol. 43, 193–197 (2012).
Sirkiä, P. M., Virolainen, M., Lehikoinen, E. & Laaksonen, T. Fluctuating selection and immigration as determinants of the phenotypic composition of a population. Oecologia 173, 305–317 (2013).
Lehtonen, P. K. et al. Geographic patterns of genetic differentiation and plumage colour variation are different in the pied flycatcher (Ficedula hypoleuca). Mol. Ecol. 18, 4463–4476 (2009).
Visser, M. E., Holleman, L. J. M. & Gienapp, P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 (2006).
Thomas, D. W., Blondel, J., Perret, P., Lambrechts, M. M. & Speakman, J. R. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600 (2001).
Tomotani, B. M., Gienapp, P., Beersma, D. G. M. & Visser, M. E. Climate change relaxes the time constraints for late-born offspring in a long-distance migrant. Proc. R. Soc. B 283, 20161366 (2016).
Both, C., Bijlsma, R. G. & Ouwehand, J. Repeatability in spring arrival dates in pied flycatchers varies among years and sexes. Ardea 104, 3–21 (2016).
Pulido, F., Berthold, P., Mohr, G. & Querner, U. Heritability of the timing of autumn migration in a natural bird population. Proc. R. Soc. B 268, 953–959 (2001).
Gwinner, E. Circannual clocks in avian reproduction and migration. Ibis 138, 47–63 (1996).
Nicolaus, M., Ubels, R. & Both, C. Eco-evolutionary consequences of dispersal syndromes during colonization in a passerine bird. Am. Nat. 201, 523–536 (2023).
Van Der Jeugd, H. P. & McCleery, R. Effects of spatial autocorrelation, natal philopatry and phenotypic plasticity on the heritability of laying date. J. Evol. Biol. 15, 380–387 (2002).
Visser, M. E. et al. Effects of spring temperatures on the strength of selection on timing of reproduction in a long-distance migratory bird. PLoS Biol. 13, 1–17 (2015).
Potti, J. Arrival time from spring migration in male pied flycatchers: individual consistency and familial resemblance. Condor 100, 702–708 (1998).
Tarka, M., Hansson, B. & Hasselquist, D. Selection and evolutionary potential of spring arrival phenology in males and females of a migratory songbird. J. Evol. Biol. 28, 1024–1038 (2015).
Kruuk, L. E. B. & Hadfield, J. D. How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903 (2007).
Brown, C. R. & Brown, M. B. Weather-mediated natural selection on arrival time in cliff swallows (Petrochelidon pyrrhonota). Behav. Ecol. Sociobiol. 47, 339–345 (2000).
Källander, H. et al. Variation in laying date in relation to spring temperature in three species of tits (Paridae) and pied flycatchers Ficedula hypoleuca in southernmost Sweden. J. Avian Biol. 48, 83–90 (2017).
Charmantier, A. et al. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–804 (2008).
Husby, A., Gustafsson, L. & Qvarnström, A. Low genetic variance in the duration of the incubation period in a collared flycatcher (Ficedula albicollis) population. Am. Nat. 179, 132–136 (2012).
Helm, B., Doren, B. M., Van, Hoffmann, D. & Hoffmann, U. Evolutionary response to climate change in migratory pied flycatchers. Curr. Biol. 29, 3714–3719 (2019).
Paradis, E., Baillie, S. R., Sutherland, W. J. & Gregory, R. D. Patterns of natal and breeding dispersal in birds. J. Anim. Ecol. 67, 518–536 (1998).
Youngflesh, C. et al. Migratory strategy drives species-level variation in bird sensitivity to vegetation green-up. Nat. Ecol. Evol. 5, 987–994 (2021).
Samplonius, J. M. & Both, C. Climate change may affect fatal competition between two bird species. Curr. Biol. 29, 327–331 (2019).
Bauer, S., McNamara, J. M. & Barta, Z. Environmental variability, reliability of information and the timing of migration. Proc. R. Soc. B 287, 20200622 (2020).
Studds, C. E., Kyser, T. K. & Marra, P. P. Natal dispersal driven by environmental conditions interacting across the annual cycle of a migratory songbird. Proc. Natl Acad. Sci. USA 105, 2929–2933 (2008).
Alberto, F. J. et al. Potential for evolutionary responses to climate change—evidence from tree populations. Glob. Chang. Biol. 19, 1645–1661 (2013).
Loonstra, J. A. H., Verhoeven, M. A., Both, C. & Piersma, T. Translocation of shorebird siblings shows intraspecific variation in migration routines to arise after fledging. Curr. Biol. 33, 2535–2540.e3 (2023).
Madsen, J. et al. Rapid formation of new migration route and breeding area by Arctic geese. Curr. Biol. 33, 1162–1170.e4 (2023).
Reid, J. M. et al. Immigration counter‐acts local micro‐evolution of a major fitness component: migration–selection balance in free‐living song sparrows. Evol. Lett. 5, 48–60 (2021).
Visser, M. E. & Gienapp, P. Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 3, 879–885 (2019).
Møller, A. P., Rubolini, D. & Lehikoinen, E. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16195–16200 (2008).
Newson, S. E. et al. Long-term changes in the migration phenology of UK breeding birds detected by large-scale citizen science recording schemes. Ibis 158, 481–495 (2016).
Rushing, C. S., Andrew Royle, J., Ziolkowski, D. J. & Pardieck, K. L. Migratory behavior and winter geography drive differential range shifts of eastern birds in response to recent climate change. Proc. Natl Acad. Sci. USA 117, 12897–12903 (2020).
Lamers, K. P., Nicolaus, M., Rakhimberdiev, E., Nilsson, J. & Both, C. Descriptive and experimental evidence for timing‐mediated polygyny risk in a pied flycatcher Ficedula hypoleuca population. J. Avian Biol. 51, 1–12 (2020).
Skjelseth, S., Ringsby, T. H., Tufto, J., Jensen, H. & Sæther, B. E. Dispersal of introduced house sparrows Passer domesticus: an experiment. Proc. R. Soc. B 274, 1763–1771 (2007).
Krogstad, S., Sæther, B. E. & Solberg, E. J. Environmental and genetic determinants of reproduction in the house sparrow: a transplant experiment. J. Evol. Biol. 9, 979–991 (1996).
Germain, M., Pärt, T. & Doligez, B. Lower settlement following a forced displacement experiment: nonbreeding as a dispersal cost in a wild bird? Anim. Behav. 133, 109–121 (2017).
Samplonius, J. M., Kappers, E. F., Brands, S., Both, C. & Phillimore, A. Phenological mismatch and ontogenetic diet shifts interactively affect offspring condition in a passerine. J. Anim. Ecol. 85, 1255–1264 (2016).
Ouwehand, J., Burger, C. & Both, C. Shifts in hatch dates do not provide pied flycatchers with a rapid ontogenetic route to adjust offspring time schedules to climate change. Funct. Ecol. 31, 1–11 (2017).
Visser, M. E. et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B 270, 367–372 (2003).
Both, C., Burger, C., Ouwehand, J., Samplonius, J. M. & Bijlsma, R. G. Delayed age at first breeding and experimental removals show large non-breeding surplus in pied flycatchers. Ardea 105, 43–60 (2017).
Ouwehand, J. & Both, C. Alternate non-stop migration strategies of pied flycatchers to cross the Sahara desert. Biol. Lett. 12, 20151060 (2016).
Bates, D., Maechler, M. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
R Core Team. R: A Language and Environment for Statistical Computing (Royal Foundation for Statistical Computing, 2020).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-theoretic Approach (Springer, 2002).
Acknowledgements
We are immensely grateful to the many researchers whose input and immense dedication collecting data in the field made this study possible. We particularly thank A. Maurukaite, J. Allain, R. Buhus, R. Engert, L. Jhaveri, H. Roodenrijs, T. Vamos, L. McBride, T. Micallef, E. Zuidema, K. Brouwer, A. Cillard, S. Barrault, J. Bliss, X. Wang and R. Ubels. We also thank S. Martens, who contributed unpublished data from his German study population. Staatsbosbeheer, Natuurmonumenten and Vombverket kindly allowed us to work on their properties. We thank B. Sheldon and P. Edelaar for commenting on an earlier draft. This publication and one of the field seasons was supported by a contribution to K.P.L. from the Meester Prikkebeen Fonds, managed by the Prins Bernhard Cultuurfonds, which oversees more than 450 CultuurFondsen. This study was funded by the Netherlands Organization for Scientific Research (NWO-ALW to C.B. ALWOP.171).
Author information
Authors and Affiliations
Contributions
All authors (K.P.L., M.N., J.-Å.N. and C.B.) contributed in conceiving the study and designing the experiments. K.P.L. collected the data in Sweden, and J.-Å.N. contributed vital support. C.B. and M.N. collected data in the Netherlands. K.P.L. analysed the data. K.P.L. and C.B. wrote the first draft. All authors (K.P.L., M.N., J.-Å.N. and C.B.) revised and commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Aneta Arct, Carlos Camacho, Anne Goodenough and Albert Phillimore for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Timing of the Swedish unmanipulated pied flycatcher population compared with the Dutch population and experimental translocation groups.
(A) Mean first egg laying dates of unmanipulated pied flycatcher females in the Dutch and Swedish study sites. Data are shown for the experimental years 2017, 2018 and 2019. The error bars represent ± 2 s.e. and sample sizes and mean values are given in text. (B) Centred first egg laying dates of females of the different translocation treatments breeding in Vomb, Sweden. The bars are stacked in order: Dutch females translocated to Sweden (red), control translocations (blue) and unmanipulated females (grey).
Extended Data Fig. 2 Mean number of recruits per nest of pied flycatcher females of Dutch origin, measured as locally returning offspring in the two years after the translocation treatment of a nest.
This figure is equivalent to Fig. 2 in the main text: whilst there we examined the fitness of Dutch birds translocated to Sweden in comparison to the new local (Swedish) population, we here display their fitness compared to conspecifics at their location of origin. Data are plotted as four hatching date quantiles per treatment. Point size is scaled to the square root of the sample size of the quantile. The line represents averaged model estimates of a model including hatching date plotted over the range of observed values, with separate intercepts for the treatment groups because the Dutch translocated group had a significantly higher intercept (Extended Data Table 3CD). For each year, hatching date is centred to the median hatching date of unmanipulated females in the Netherlands (and hence the hatching dates differ from Fig. 2). Apart from the two treatment groups that were translocated (either from The Netherlands to Sweden (red dots; n = 20), or within the Netherlands (orange triangles; n = 20)) we also plot the unmanipulated Dutch nests (grey squares; n = 618).
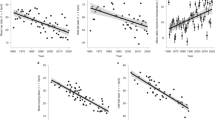
Extended Data Fig. 3 Mean laying dates of the first egg over the years for pied flycatchers breeding in Vomb, Sweden.
Open symbols represent data we collected of unmanipulated females (2017-2020), while the closed symbols are data of the period 1971-2011 from Källander et al. (2017). The slope of the year-model is represented by the solid line (see Extended Data Table 7).
Supplementary information
Source data
Source Data Fig. 1
Laying dates of translocated females and their release date from the aviary. Same data as for Extended Data Table 2.
Source Data Fig. 2
Number of recruiting offspring after 2 years for females of the three translocation treatments (translocated to Sweden, within Sweden or unmanipulated in Sweden) and their hatching date (centred to the local Swedish population). Same data as for Extended Data Table 3a,b.
Source Data Fig. 3
Data for the timing of spring annual cycle activities (spring departure, spring arrival and laying date in separate files) for ‘nature’ comparisons (comparison between the groups of different genetic origin) and ‘nurture’ comparisons (comparisons between the Netherlands and Sweden for birds of genetically Dutch origin). Dates are in aprildate (1 = 1 April). Age is the number of years a bird is old (hatching in 2019 and spring arrival recorded in 2020 = 1 year old), and sex is female (1) or male (2). Same data as for Extended Data Tables 4 and 5.
Source Data Extended Data Table 1
Settlement rate data for females translocated within the Netherlands (NL_C), within Sweden (SE_C) or from the Netherlands to Sweden (SE_T). Settled = 1 and not settled = 0. The stage at which the female was translocated is included (NB, when nestbuilding; IN, at the start of incubation).
Source Data Extended Data Table 2
Laying dates of translocated females and their release date from the aviary. Same data as for Fig. 1.
Source Data Extended Data Table 3
a,b, Number of recruiting offspring after 2 years for females of the three translocation treatments (translocated to Sweden, within Sweden or unmanipulated in Sweden) and their hatching date (centred to the local Swedish population). Same data as for Fig. 2. c,d, Number of recruiting offspring after 2 years for females of the three translocation treatments (translocated to Sweden, within the Netherlands or unmanipulated in the Netherlands) and their hatching date (centred to the original Dutch population). Same data as for Extended Data Fig. 2.
Source Data Extended Data Table 4
Data for the timing of spring annual cycle activities (spring departure, spring arrival and laying date in separate files) for ‘nature’ comparisons (comparison between the groups of different genetic origin) and ‘nurture’ comparisons (comparisons between the Netherlands and Sweden for birds of genetically Dutch origin). Dates are in aprildate (1 = 1 April). Age is the number of years a bird is old (hatching in 2019 and spring arrival recorded in 2020 = 1 year old), and sex is female (1) or male (2). Same data as for Extended Data Table 5 and Fig. 3.
Source Data Extended Data Table 5
Data for the timing of spring annual cycle activities (spring departure, spring arrival and laying date in separate files) for ‘nature’ comparisons (comparison between the groups of different genetic origin) and ‘nurture’ comparisons (comparisons between the Netherlands and Sweden for birds of genetically Dutch origin). Dates are in aprildate (1 = 1 April). Age is the number of years a bird is old (hatching in 2019 and spring arrival recorded in 2020 = 1 year old), and sex is female (1) or male (2). Same data as for Extended Data Table 4 and Fig. 3.
Source Data Extended Data Table 6
Recruitment rate data for pied flycatchers of different genetic origins born in the Swedish common garden experiment. Birds are of 0%, 50% or 100% Dutch genetic origin and either were caught again at the study site within the two field seasons after the year of hatching (1) or were not caught again (0). The nest box and year combination is included to indicate family, as well as the total number of young hatched in that nest and the hatching date (centred to the median hatching date of the broods of unmanipulated Swedish females).
Source Data Extended Data Table 7
Laying data from pied flycatchers in the Vomb region from the Källander paper37. With added new years from our own field seasons. Temperature data were acquired in the same way as described in Källander et al.37, from the Swedish Meteorological and Hydrological Institute’s Lund weather station. Same data as for Extended Data Fig3.
Source Data Extended Data Fig. 1
a, Laying dates for unmanipulated Swedish and unmanipulated Dutch females in the years of our female translocation experiment. b, Laying dates (centred for each year to unmanipulated females) of females translocated from the Netherlands to Sweden, translocated within Sweden and unmanipulated.
Source Data Extended Data Fig. 2
Number of recruiting offspring after 2 years for females of the three translocation treatments (translocated to Sweden, within the Netherlands or unmanipulated in the Netherlands) and their hatching date (centred to the original Dutch population). Same data as for Extended Data Table 3c,d.
Source Data Extended Data Fig. 3
Laying data from pied flycatchers in the Vomb region from the Källander paper37. With added new years from our own field seasons. Temperature data were acquired in the same way as described in Källander et al.37, from the Swedish Meteorological and Hydrological Institute’s Lund weather station. Same data as for Extended Data Table 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lamers, K.P., Nilsson, JÅ., Nicolaus, M. et al. Adaptation to climate change through dispersal and inherited timing in an avian migrant. Nat Ecol Evol 7, 1869–1877 (2023). https://doi.org/10.1038/s41559-023-02191-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02191-w