Abstract
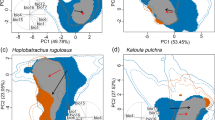
Global change is causing an unprecedented restructuring of ecosystems, with the spread of invasive species being a key driver. While population declines of native species due to invasives are well documented, much less is known about whether new biotic interactions reshape niches of native species. Here we quantify geographic range and realized-niche contractions in Australian frog species following the introduction of amphibian chytrid fungus Batrachochytrium dendrobatidis, a pathogen responsible for catastrophic amphibian declines worldwide. We show that chytrid-impacted species experienced proportionately greater contractions in niche breadth than geographic distribution following chytrid emergence. Furthermore, niche contractions were directional, with contemporary distributions of chytrid-impacted species characterized by higher temperatures, lower diurnal temperature range, higher precipitation and lower elevations. Areas with these conditions may enable host persistence with chytrid through lower pathogenicity of the fungus and/or greater demographic resilience. Nevertheless, contraction to a narrower subset of environmental conditions could increase host vulnerability to other threatening processes and should be considered in assessments of extinction risk and during conservation planning. More broadly, our results emphasize that biotic interactions can strongly shape species realized niches and that large-scale niche contractions due to new species interactions—particularly emerging pathogens—could be widespread.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw niche variable values associated with the occurrence records of each species can be accessed at the Zenodo repository: https://doi.org/10.5281/zenodo.8088601. Metadata for each species and results for all niche analyses are in the Supplementary Data. Note, occurrence records for sensitive species are buffered to 10 km in most public databases (non-buffered records were obtained for these species under license for this work).
Code availability
R code to replicate the hypervolume, geographic distribution (EOO), niche metrics calculations and statistical analyses can be accessed at the Zenodo repository: https://doi.org/10.5281/zenodo.8088601
References
Wisz, M. S. et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. 88, 15–30 (2013).
Carscadden, K. A. et al. Niche breadth: causes and consequences for ecology, evolution, and conservation. Q. Rev. Biol. 95, 179–214 (2020).
Sexton, J. P., Montiel, J., Shay, J. E., Stephens, M. R. & Slatyer, R. A. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 48, 183–206 (2017).
Sexton, J. P., McIntyre, P. J., Angert, A. L. & Rice, K. J. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436 (2009).
Hortal, J. et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549 (2015).
Díaz, S. et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366, eaax3100 (2019).
Louthan, A. M., Doak, D. F. & Angert, A. L. Where and when do species interactions set range limits? Trends Ecol. Evol. 30, 780–792 (2015).
Araújo, M. B. & Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753 (2007).
Anderson, R. P. When and how should biotic interactions be considered in models of species niches and distributions? J. Biogeogr. 44, 8–17 (2017).
Clavero, M. & García-Berthou, E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110 (2005).
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G. & Dickman, C. R. Invasive predators and global biodiversity loss. Proc. Natl Acad. Sci. USA 113, 11261–11265 (2016).
Guisan, A., Petitpierre, B., Broennimann, O., Daehler, C. & Kueffer, C. Unifying niche shift studies: insights from biological invasions. Trends Ecol. Evol. 29, 260–269 (2014).
Scheele, B. C., Foster, C. N., Banks, S. C. & Lindenmayer, D. B. Niche contractions in declining species: mechanisms and consequences. Trends Ecol. Evol. 32, 346–355 (2017).
Holt, R. D. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19659–19665 (2009).
Mace, G. M. et al. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 22, 1424–1442 (2008).
Fisher, M. C. & Garner, T. W. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 18, 332–343 (2020).
Scheele, B. C. et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463 (2019).
Skerratt, L. F. et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4, 125–134 (2007).
Scheele, B. C. et al. After the epidemic: ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis. Biol. Conserv. 206, 37–46 (2017).
Brannelly, L. A. et al. Mechanisms underlying host persistence following amphibian disease emergence determine appropriate management strategies. Ecol. Lett. 24, 130–148 (2021).
Fisher, M. C., Pasmans, F. & Martel, A. Virulence and pathogenicity of chytrid fungi causing amphibian extinctions. Annu. Rev. Microbiol. 75, 673–693 (2021).
Skerratt, L. F. et al. Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildl. Res. 43, 105–120 (2016).
Gillespie, G. R. et al. Status and priority conservation actions for Australian frog species. Biol. Conserv. 247, 108543 (2020).
Colwell, R. K. & Rangel, T. F. Hutchinson’s duality: the once and future niche. Proc. Natl Acad. Sci. USA 106, 19651–19658 (2009).
Peterson, A. T. et al. Ecological Niches and Geographic Distributions (Princeton Univ. Press, 2011).
Soberón, J. & Nakamura, M. Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl Acad. Sci. USA 106, 19644–19650 (2009).
Murray, K. A. et al. Assessing spatial patterns of disease risk to biodiversity: implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. J. Appl. Ecol. 48, 163–173 (2011).
Granados-Martínez, S., Zumbado-Ulate, H., Searle, C. L., Oliveira, B. F. & García-Rodríguez, A. Niche contraction of an endangered frog driven by the amphibian chytrid fungus. EcoHealth 18, 134–144 (2021).
Puschendorf, R. et al. Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: proposing climatic refuges as a conservation tool. Divers. Distrib. 15, 401–408 (2009).
Scheele, B. C. et al. Living with the enemy: facilitating amphibian coexistence with disease. Biol. Conserv. 236, 52–59 (2019).
Heard, G. W. et al. Refugia and connectivity sustain amphibian metapopulations afflicted by disease. Ecol. Lett. 18, 853–863 (2015).
Puschendorf, R. et al. Environmental refuge from disease-driven amphibian extinction. Conserv. Biol. 25, 956–964 (2011).
Olson, D. H., Ronnenberg, K. L., Glidden, C. K., Christiansen, K. R. & Blaustein, A. R. Global patterns of the fungal pathogen Batrachochytrium dendrobatidis support conservation urgency. Front. Vet. Sci. 8, 685877 (2021).
Morrison, C. & Hero, J. M. Geographic variation in life-history characteristics of amphibians: a review. J. Anim. Ecol. 72, 270–279 (2003).
Caruso, N. M. & Rissler, L. J. Demographic consequences of climate variation along an elevational gradient for a montane terrestrial salamander. Popul. Ecol. 61, 171–182 (2019).
Hardy, B. M. et al. Compensatory recruitment unlikely in high‐elevation amphibian populations challenged with disease. J. Appl. Ecol. 60, 121–131 (2022).
Scheele, B. C. et al. Landscape context influences chytrid fungus distribution in an endangered European amphibian. Anim. Conserv. 18, 480–488 (2015).
Maher, S. P., Ellis, C., Gage, K. L., Enscore, R. E. & Peterson, A. T. Range-wide determinants of plague distribution in North America. Am. J. Tropical Med. Hyg. 83, 736 (2010).
Rahbek, C. et al. Humboldt’s enigma: what causes global patterns of mountain biodiversity? Science 365, 1108–1113 (2019).
Cardillo, M., Dinnage, R. & McAlister, W. The relationship between environmental niche breadth and geographic range size across plant species. J. Biogeogr. 46, 97–109 (2019).
Cummins, D., Kennington, W. J., Rudin‐Bitterli, T. & Mitchell, N. J. A genome‐wide search for local adaptation in a terrestrial‐breeding frog reveals vulnerability to climate change. Glob. Change Biol. 25, 3151–3162 (2019).
Bachmann, J. C. & Van Buskirk, J. Adaptation to elevation but limited local adaptation in an amphibian. Evolution 75, 956–969 (2021).
Reside, A. E. et al. Beyond the model: expert knowledge improves predictions of species’ fates under climate change. Ecol. Appl. 29, e01824 (2019).
Sopniewski, J., Scheele, B. C. & Cardillo, M. Predicting the distribution of Australian frogs and their overlap with Batrachochytrium dendrobatidis under climate change. Divers. Distrib. 28, 1255–1268 (2022).
Savage, A. E. & Zamudio, K. R. MHC genotypes associate with resistance to a frog-killing fungus. Proc. Natl Acad. Sci. USA 108, 16705–16710 (2011).
Scheele, B. C. et al. Disease-associated change in an amphibian life-history trait. Oecologia 184, 825–833 (2017).
Roznik, E. A., Sapsford, S. J., Pike, D. A., Schwarzkopf, L. & Alford, R. A. Condition-dependent reproductive effort in frogs infected by a widespread pathogen. Proc. R. Soc. Lond. B 282, 20150694 (2015).
Stevenson, L. A. et al. Variation in thermal performance of a widespread pathogen, the amphibian chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 8, e73830 (2013).
van Riper, C. III, van Riper, S. G., Goff, M. L. & Laird, M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 56, 327–344 (1986).
Burke, K. L. Niche contraction of American chestnut in response to chestnut blight. Can. J. For. Res. 42, 614–620 (2012).
Doherty, T. S., Dickman, C. R., Nimmo, D. G. & Ritchie, E. G. Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances. Biol. Conserv. 190, 60–68 (2015).
The IUCN Red List of Threatened Species. Version 2021–3 (IUCN, accessed December 2021); https://www.iucnredlist.org
Breiner, F. T., Guisan, A., Nobis, M. P. & Bergamini, A. Including environmental niche information to improve IUCN Red List assessments. Divers. Distrib. 23, 484–495 (2017).
von Takach, B., Scheele, B. C., Moore, H., Murphy, B. P. & Banks, S. C. Patterns of niche contraction identify vital refuge areas for declining mammals. Divers. Distrib. 26, 1467–1482 (2020).
Atlas of Living Australia. Occurrence Records Download on 2021-11-10 (ALA, 2021); https://doi.org/10.26197/ala.ce7f5cdf-5d26-4dd2-9f53-222586068405
GBIF Occurrence Download 10 November 2021 (GBIF, 2021); https://doi.org/10.15468/dl.eurhxu
Rowley, J. J. & Callaghan, C. T. The FrogID dataset: expert-validated occurrence records of Australia’s frogs collected by citizen scientists. ZooKeys 912, 139 (2020).
Hoskin, C., Grigg, G., Stewart, D. & McDonald, S. Frogs of Australia—A Complete Electronic Field Guide to Australian Frogs (Ug Media, 2015); https://espace.library.uq.edu.au/view/UQ:375857
Bivand, R. et al. rgdal: bindings for the geospatial data abstraction library. R package version 1.4-8 (2019).
Bivand, R., Rundel, C. & Pebesma, E. rgeos: interface to geometry engine-open source (GEOS). R package version 0.3-26 (2017).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
Anstis, M. Tadpoles and Frogs of Australia (New Holland Publishers, 2013).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Naimi, B., Hamm, N. A., Groen, T. A., Skidmore, A. K. & Toxopeus, A. G. Where is positional uncertainty a problem for species distribution modelling? Ecography 37, 191–203 (2014).
Martínez‐Freiría, F., Tarroso, P., Rebelo, H. & Brito, J. C. Contemporary niche contraction affects climate change predictions for elephants and giraffes. Divers. Distrib. 22, 432–444 (2015).
Monsarrat, S., Novellie, P., Rushworth, I. & Kerley, G. Shifted distribution baselines: neglecting long-term biodiversity records risks overlooking potentially suitable habitat for conservation management. Phil. Trans. R. Soc. B 374, 20190215 (2019).
Blonder, B. et al. New approaches for delineating n‐dimensional hypervolumes. Methods Ecol. Evol. 9, 305–319 (2018).
Blonder, B., Lamanna, C., Violle, C. & Enquist, B. J. The n‐dimensional hypervolume. Glob. Ecol. Biogeogr. 23, 595–609 (2014).
Pack, K. E., Mieszkowska, N. & Rius, M. Rapid niche shifts as drivers for the spread of a non‐indigenous species under novel environmental conditions. Divers. Distrib. 28, 596–610 (2022).
Pili, A. N., Tingley, R., Sy, E. Y., Diesmos, M. L. L. & Diesmos, A. C. Niche shifts and environmental non-equilibrium undermine the usefulness of ecological niche models for invasion risk assessments. Sci. Rep. 10, 7972 (2020).
Mammola, S. & Cardoso, P. Functional diversity metrics using kernel density n‐dimensional hypervolumes. Methods Ecol. Evol. 11, 986–995 (2020).
Pateiro-Lopez, B., Rodriguez-Casal, A. & Pateiro-Lopez, M. B. alphahull: generalization of the convex hull of a sample of points in the plane. R package version 2 (2016).
Pebesma, E. et al. sp: classes and methods for spatial data. R package version 1.4-7 (2023).
Hijmans, R. J. et al. raster: geographic data analysis and modeling. R package version 3.5-15 (2021).
Mapping Standards and Data Quality for the IUCN Red List Spatial Data (IUCN, 2021); https://www.iucnredlist.org/resources/mappingstandards#:~:text=The%20Mapping%20Standards%20and%20Data,version%201.19%20 (May%202021)
Carvalho, J. C. & Cardoso, P. Decomposing the causes for niche differentiation between species using hypervolumes. Front. Ecol. Evol. 8, 243 (2020).
Kellner, K. jagsUI: a wrapper around rjags to streamline JAGS analyses. R package version 1.5.2 (2019).
Acknowledgements
B.C.S. was supported by The Australian Research Council through a Discovery Early Career Research Award (DE200100121). FrogID data were used with the permission of the Australian Museum.
Author information
Authors and Affiliations
Contributions
B.C.S. secured funding. B.C.S., R.P.D., G.W.H., M.C. and J.S. developed the study questions and design. B.C.S., G.W.H., G.R.G., C.J.H., M.M., D.N. and J.J.L.R. checked species records. J.S. and R.P.D. led analyses. B.C.S. wrote the first draft of the paper and all authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Luis Escobar, Adrián Garcia Rodriguez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–57.
Supplementary Data
Metadata on species included in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scheele, B.C., Heard, G.W., Cardillo, M. et al. An invasive pathogen drives directional niche contractions in amphibians. Nat Ecol Evol 7, 1682–1692 (2023). https://doi.org/10.1038/s41559-023-02155-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02155-0
This article is cited by
-
Chytrid invasion drives frog redistributions
Nature Ecology & Evolution (2023)