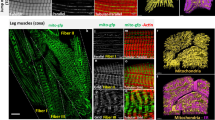
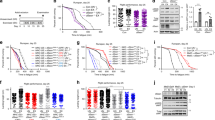
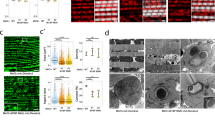
Abstract
Ageing plasticity represents the flexibility of the ageing process in response to non-genetic factors, occurring commonly in animals. However, the regulatory mechanisms underlying ageing plasticity are largely unclear. The density-dependent polyphenism of locusts, Locusta migratoria, displays dramatic lifespan divergence between solitary and gregarious phases, providing a useful system for studying ageing plasticity. Here, we found that gregarious locusts displayed faster locomotor deficits and increased muscle degeneration on ageing than solitary locusts. Comparative transcriptome analysis in flight muscles revealed significant differences in transcriptional patterns on ageing between two phases. RNA interference screening showed that the knockdown of the upregulated PLIN2 gene significantly relieved the ageing-related flight impairments in gregarious locusts. Mechanistically, the gradual upregulation of PLIN2 could induce the accumulation of ectopic lipid droplets and triacylglycerols in flight muscles during the ageing process. Further experiments suggested that ectopic lipid accumulation led to an ageing-related β-oxidation decline through limiting fatty acid transport and content. These findings reveal the key roles of lipid metabolism in the differences of muscle ageing between solitary and gregarious locusts and provide a potential mechanism underlying environment-induced muscle ageing plasticity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data and materials generated in this study are available in the main text, supplementary materials and source data. The RNA-seq data were deposited at the NCBI Sequence Read Archive under BioProject no. PRJNA562411 and the National Genomics Data Center under BioProject no. CRA006718. Source data are provided with this paper.
References
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Jones, O. R. et al. Diversity of ageing across the tree of life. Nature 505, 169–173 (2014).
Guo, S., Wang, X. & Kang, L. Special significance of non-Drosophila insects in aging. Front. Cell Dev. Biol. 8, 576571 (2020).
Keller, L. & Jemielity, S. Social insects as a model to study the molecular basis of ageing. Exp. Gerontol. 41, 553–556 (2006).
Korb, J. & Heinze, J. Ageing and sociality: why, when and how does sociality change ageing patterns? Philos. Trans. R. Soc. Lond. B Biol. Sci. 376, 20190727 (2021).
Williams, P. D., Day, T., Fletcher, Q. & Rowe, L. The shaping of senescence in the wild. Trends Ecol. Evol. 21, 458–463 (2006).
Lucas, E. R. & Keller, L. The co-evolution of longevity and social life. Funct. Ecol. 34, 76–87 (2020).
Kapahi, P., Kaeberlein, M. & Hansen, M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev. 39, 3–14 (2017).
Ruan, H. & Wu, C.-F. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proc. Natl Acad. Sci. USA 105, 7506–7510 (2008).
Galimov, E. R. & Gems, D. Death happy: adaptive ageing and its evolution by kin selection in organisms with colonial ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 376, 20190730 (2021).
Cartee, G. D., Hepple, R. T., Bamman, M. M. & Zierath, J. R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 23, 1034–1047 (2016).
Pener, M. P. & Simpson, S. J. Locust phase polyphenism: an update. Adv. Insect Physiol. 36, 1–272 (2009).
Wang, X. & Kang, L. Molecular mechanisms of phase change in locusts. Annu. Rev. Entomol. 59, 225–244 (2014).
Guo, X. et al. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 584, 584–588 (2020).
Wei, J. et al. Phenylacetonitrile in locusts facilitates an antipredator defense by acting as an olfactory aposematic signal and cyanide precursor. Sci. Adv. 5, eaav5495 (2019).
Guo, W. et al. CSP and takeout genes modulate the switch between attraction and repulsion during behavioral phase change in the migratory locust. PLoS Genet. 7, e1001291 (2011).
Yang, M. et al. A β-carotene-binding protein carrying a red pigment regulates body-color transition between green and black in locusts. eLife 8, e41362 (2019).
Du, B., Ding, D., Ma, C., Guo, W. & Kang, L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc. Natl Acad. Sci. USA 119, e2115753118 (2022).
Boerjan, B. et al. Locust phase polyphenism: does epigenetic precede endocrine regulation? Gen. Comp. Endocrinol. 173, 120–128 (2011).
Guo, S. et al. Aging features of the migratory locust at physiological and transcriptional levels. BMC Genomics 22, 257 (2021).
Gordon, S. D. & Windmill, J. F. C. Hearing ability decreases in ageing locusts. J. Exp. Biol. 218, 1990–1994 (2015).
Parle, E. & Taylor, D. The effect of aging on the mechanical behaviour of cuticle in the locust Schistocerca gregaria. J. Mech. Behav. Biomed. Mater. 68, 247–251 (2017).
Liu, H., Li, K. B., Yin, J., Du, G. L. & Cao, Y. Z. Ultrastructure of the flight muscle of female adults in the gregarious phase and solitary phase of the oriental migratory locust, Locusta migratoria manilensis (Meyen) (Orthoptera: Acrididae). Acta Entomol. Sin. 51, 1033–1038 (2008).
Hou, L., Guo, S., Ding, D., Du, B. & Wang, X. Neuroendocrinal and molecular basis of flight performance in locusts. Cell. Mol. Life Sci. 79, 325 (2022).
de Magalhães, J. P., Curado, J. & Church, G. M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 25, 875–881 (2009).
Lin, Z. et al. Simultaneous dimension reduction and adjustment for confounding variation. Proc. Natl Acad. Sci. USA 113, 14662–14667 (2016).
Hou, L. et al. Neuropeptide ACP facilitates lipid oxidation and utilization during long-term flight in locusts. eLife 10, e65279 (2021).
Bi, J. et al. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J. Cell Sci. 125, 3568–3577 (2012).
Zahn, J. M. et al. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2, e115 (2006).
Palikaras, K. et al. Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans. J. Lipid Res. 58, 72–80 (2017).
Golden, J. W. & Riddle, D. L. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218, 578–580 (1982).
Joshi, A. & Mueller, L. D. Adult crowding effects on longevity in Drosophila melanogaster: increase in age-independent mortality. Curr. Sci. 72, 255–260 (1997).
Lin, W. et al. Long-term crowding stress causes compromised nonspecific immunity and increases apoptosis of spleen in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 80, 540–545 (2018).
Lin, E.-J. D. et al. Social overcrowding as a chronic stress model that increases adiposity in mice. Psychoneuroendocrinology 51, 318–330 (2015).
Yan, Y. et al. HDAC6 suppresses age-dependent ectopic fat accumulation by maintaining the proteostasis of PLIN2 in Drosophila. Dev. Cell 43, 99–111 (2017).
Delmonico, M. J. et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 90, 1579–1585 (2009).
Correa-de-Araujo, R. et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front. Physiol. 11, 963 (2020).
Bergman, B. C. et al. Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans. Am. J. Physiol. Endocrinol. Metab. 314, E152–E164 (2018).
Chee, C. et al. Relative contribution of intramyocellular lipid to whole-body fat oxidation is reduced with age but subsarcolemmal lipid accumulation and insulin resistance are only associated with overweight individuals. Diabetes 65, 840–850 (2016).
Cree, M. G. et al. Intramuscular and liver triglycerides are increased in the elderly. J. Clin. Endocrinol. Metab. 89, 3864–3871 (2004).
Daemen, S. et al. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol. Metab. 17, 71–81 (2018).
Zhang, W., Qu, J., Liu, G.-H. & Belmonte, J. C. I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 21, 137–150 (2020).
Villeponteau, B. The heterochromatin loss model of aging. Exp. Gerontol. 32, 383–394 (1997).
Wood, J. G. et al. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc. Natl Acad. Sci. USA 113, 11277–11282 (2016).
Benayoun, B. A., Pollina, E. A. & Brunet, A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 16, 593–610 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Wang, X. et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 5, 2957 (2014).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Yang, P., Hou, L., Wang, X. & Kang, L. Core transcriptional signatures of phase change in the migratory locust. Protein Cell 10, 883–901 (2019).
Beissbarth, T. & Speed, T. P. GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics 20, 1464–1465 (2004).
Acknowledgements
We thank J. Ge and D. Ding for their comments and suggestions to improve the manuscript, and P. Yang and B. Du for assistance with the Python and R software. We thank S. Liu, W. Cui and J. Yu for preparing the locusts. We thank iPheome (Yunpukang) Biotechnology for the lipidomic analyses. This work was supported by the National Key R&D Program of China no. 2022YFD1400500, the National Natural Science Foundation of China (grant nos. 31930012, 32088102 and 32100388), the China Postdoctoral Science Foundation (no. 2021M703204) and Youth Innovation Promotion Association CAS (no. 2021079).
Author information
Authors and Affiliations
Contributions
S.G., L.K. and X.W. designed the research. S.G., L.H. and L.D. performed the research. S.G. and X.N. contributed the analytical tools. S.G. and X.W. analysed the data. S.G., L.H., L.K. and X.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Johan Auwerx and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Abundance detection of total ROS in flight muscles of solitary and gregarious locusts during ageing.
n = 6 for the comparison between 14-D and 42-D solitary locusts and between 14-D and 28-D gregarious locusts, n = 7 for the comparison between 28-D solitary and 28-D gregarious locusts. Data are mean ± s.e.m. Significant differences are analysed using two-tailed unpaired t test.
Extended Data Fig. 2 RNAi efficiency of four ageing-related genes in muscles.
n = 6 for all conditions. Data are mean ± s.e.m. Significant differences are analysed using two-tailed unpaired t test.
Extended Data Fig. 3 Expression levels of PLIN2 during ageing in flight muscles of solitary and gregarious locusts at different population densities.
n = 5 for 28-d gregarious locusts (rearing density = 200 individuals per cage), and n = 6 for all other conditions. Data are mean ± s.e.m. Different letters corresponding to the population density indicate statistically significant differences across age groups using one-way ANOVA (Tukey’s multiple comparisons test, P < 0.05).
Extended Data Fig. 4 Lifespan of gregarious (G) and solitary (S) locust males after PLIN2 knockdown.
Blue and red lines represent the survival curves of dsGFP and dsPLIN2-injected locusts, respectively. n = 33 (dsGFP, gregarious locusts), 33 (dsPLIN2, gregarious locusts), 36 (dsGFP, solitary locusts) and 36 (dsPLIN2, solitary locusts). The P-value is determined by the Gehan–Breslow–Wilcoxon test (P = 0.670 for G, and 0.748 for S).
Extended Data Fig. 5 Pie chart of metabolite chemical classes structurally annotated.
In total, 593 lipid metabolites are characterised.
Extended Data Fig. 6 Gradual upregulation of PLIN2-induced ectopic lipids during ageing is closely related to muscle deficiency in gregarious locusts.
a, Measuring expression levels of PLIN2 during 21–28 D in flight muscles of gregarious locusts by qPCR. n = 5 for 14 D and 6 for all other conditions. b, LD staining and quantification in flight muscles of gregarious locusts during 21–28 D. Blue indicates cell nuclei stained by Hoechest 33342, and green indicates lipid droplets stained by BODIPYTM 493/503. Scale bars represent 50 μm. n = 5 for all conditions. c, Abundance detection of TAG in flight muscles of gregarious locusts during 21–28 d. n = 6 for all conditions. d, Detection of the TAG level, flight performance and ATP level in gregarious locusts at 14, 21, 23, 25 and 28 d after PLIN2 knockdown. For the TAG assays, n = 6 for all conditions. For the flight performance assays, n = 33 (14-d, dsGFP), 34 (14-d, dsPLIN2), 36 (21-d, dsGFP), 36 (21-d, dsPLIN2), 33 (23-d, dsGFP), 36 (23-d, dsPLIN2), 35 (25-d, dsGFP), 34 (25-d, dsPLIN2), 28 (28-d, dsGFP) and 29 (29-d, dsPLIN2). For the ATP assays, n = 6 for all conditions. For a–d, data are mean ± s.e.m. Significant differences are analysed using one-way ANOVA for multigroup comparisons (P < 0.05) (a–c) and two-tailed unpaired t test for two-group comparisons (d).
Extended Data Fig. 7 Venn diagram of DEGs after PLIN2 knockdown and during ageing in gregarious locusts.
a, Venn diagram of DEGs between upregulated genes after PLIN2 knockdown and downregulated DEGs during 21–28 d in gregarious locusts. b, Venn diagram of DEGs between downregulated genes after PLIN2 knockdown and upregulated DEGs during 21–28 d in gregarious locusts.
Extended Data Fig. 8 FABP expression in flight muscles of solitary and gregarious locusts during ageing and of gregarious locusts after PLIN2 knockdown.
a, Determination of FABP protein level by analysing band intensity on western blot images. n = 3 for S and G, 4 for PLIN2 knockdown. Significant differences are revealed using one-way ANOVA for multigroup comparisons (P < 0.05) and two-tailed unpaired t test for two-group comparison. b, Determination of FABP mRNA level by analysing RPKM value from transcriptomes. Data are mean ± s.e.m. The values that indicate statistically significant differences are the adjusted P-value with false discovery rate correction.
Extended Data Fig. 9 RNAi efficiency of ATGL in muscles.
n = 6 for both conditions. Data are mean ± s.e.m. Significant difference is analysed using two-tailed unpaired t test.
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Tables 1–6
Table 1 RPKM values of all genes and statistical analyses in the transcriptomes. Table 2 PC scores of genes based on the AC-PCA analyses. Table 3 Abundance of lipid species in lipidomes. Table 4 Abundance of fatty acid species in the LC–MS/MS analyses. Table 5 Primers used in the qPCR and RNAi experiments. Table 6 Abbreviations.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and confocal figures.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed confocal figures.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Hou, L., Dong, L. et al. PLIN2-induced ectopic lipid accumulation promotes muscle ageing in gregarious locusts. Nat Ecol Evol 7, 914–926 (2023). https://doi.org/10.1038/s41559-023-02059-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02059-z
This article is cited by
-
Adaptive physiology drives ageing plasticity in locusts
Nature Ecology & Evolution (2023)