Abstract
Human-induced environmental changes, such as the introduction of invasive species, are driving declines in the movement of nutrients across ecosystems with negative consequences for ecosystem function. Declines in nutrient inputs could thus have knock-on effects at higher trophic levels and broader ecological scales, yet these interconnections remain relatively unknown. Here we show that a terrestrial invasive species (black rats, Rattus rattus) disrupts a nutrient pathway provided by seabirds, ultimately altering the territorial behaviour of coral reef fish. In a replicated ecosystem-scale natural experiment, we found that reef fish territories were larger and the time invested in aggression lower on reefs adjacent to rat-infested islands compared with rat-free islands. This response reflected changes in the economic defendability of lower-quality resources, with reef fish obtaining less nutritional gain per unit foraging effort adjacent to rat-infested islands with low seabird populations. These results provide a novel insight into how the disruption of nutrient flows by invasive species can affect variation in territorial behaviour. Rat eradication as a conservation strategy therefore has the potential to restore species interactions via territoriality, which can scale up to influence populations and communities at higher ecological levels.
Similar content being viewed by others
Main
The movement of naturally occurring nutrients across habitats and ecosystems is a strong driver of productivity and can influence community dynamics1. Inputs from animals can contribute substantially to the nutrient budget of an ecosystem, but anthropogenic activities have reduced the movement of naturally occurring nutrients between animals to 6% of historic values2,3. While certain aspects of human-induced environmental change can increase the nutrient load to an ecosystem4, human-induced declines in the movement of nutrients can negatively impact the resources that organisms are able to exploit2. Organisms initially respond to human-induced environmental change through rapid behavioural modifications that reduce the resource demand of the organism, including changes to foraging behaviour5,6, aggression7,8 and territoriality9. Thus, reductions in nutrient inputs are predicted to alter the behaviour of higher-trophic-level organisms via cascading effects through food webs, although such connections are untested. As changes in behaviour can influence species interactions and subsequently shape ecological communities10,11, understanding behavioural responses to change is fundamental to revealing the complex ecological consequences of human-induced nutritional declines.
Nutritional resources are key drivers of territorial behaviour. The food maintenance hypothesis predicts that territory size is primarily determined by the nutritional requirements that allow organisms to meet short-term energetic needs12. An inverse relationship between resource availability and territory size13 is apparent across a range of organisms including mammals14, reptiles15, birds16 and fish17. Theory proposes that aggression is also largely determined by nutritional resources, such that the aggressive defence of territories, or territoriality, is predicted to occur only where the energetic benefits of territoriality outweigh the costs18. Under this model of economic defendability, a bell-shaped relationship between territoriality and resource level is predicted, and territoriality is only beneficial when the value of a nutritional resource is above a certain threshold value19. When nutritional resources are rare, the energetic cost of territoriality is too high. At intermediate resource levels, positive net benefits for territoriality outweigh the benefits of non-territorial behaviour, and the net pay-off for territorial behaviour reaches a peak18,20. Where resources are in excess, the highest net benefit occurs for non-territorial behaviour, as individuals can obtain resources at low cost without the need for aggression19. It is plausible that the disruption of nutrient pathways by human-induced environmental change lowers the value of nutritional resources, impacting the cost-benefit dynamics of territoriality and the subsequent territorial tendencies of individuals13,19. However, the consequences of declines in nutrient transfer on variation in territorial behaviour remains untested.
Seabirds are globally important contributors to nutrient transfer, responsible for a cascade of nutrients through terrestrial21 and marine22 ecosystems by depositing guano on islands after feeding in the open ocean. Invasive species, including black rats (Rattus rattus), disrupt this nutrient pathway by driving declines in seabird densities via predation23. Nutrient subsidies from seabirds flowing onto coral reef ecosystems result in higher nitrogen stable isotope quantities (δ15N) in algae and fish24,25, enhanced coral growth26, higher reef fish biomass27 and faster growth rate in herbivorous fishes22,28. Furthermore, the presence of invasive rats negatively impacts reef fish diversity and ecosystem function on adjacent coral reefs29. Invasive rats, therefore, have a substantial bottom-up impact on terrestrial and marine ecosystems. As behaviour is an important mediator between organisms and the environment11, the behavioural responses of reef organisms could play a mechanistic role in the ecological changes observed on coral reefs adjacent to rat-infested islands.
Here we use a control-treatment experimental design to provide a novel insight into the cascading effects of seabird nutrient subsidies on territorial behaviour and reveal how these effects are disrupted by invasive rats, using the herbivorous farmer damselfish, Plectroglyphidodon lacrymatus. We test the model of economic defendability18 and identify the role of nutritional subsidies in placing the value of resources above the critical threshold value for territoriality. We predict that the nutrient enrichment of resources by seabirds will result in higher aggression and a smaller territory size for P. lacrymatus individuals on reefs around rat-free islands compared with individuals on reefs around rat-infested islands. Using in situ observations, we link the aggression of individual fish to their territory size, and the quantity and nitrogen enrichment of their nutritional resources to show how a reduction in nutrient subsidies can drive both broad and fine-scale variability in territorial behaviour.
We studied ten islands (n = 5 rat-free islands, n = 5 rat-infested islands, Extended Data Fig. 1) across three atolls within the Chagos Archipelago, where seabird densities on rat-free islands are up to 720 times higher, and the nitrogen input provided by seabirds is 251 times greater, than around rat-infested islands22. We calculated the area of 60 damselfish territories (n = 30 around islands with seabirds, n = 30 around islands with rats), and recorded the aggressive behaviour of 57 of these 60 individuals (n = 28 around islands with seabirds, n = 29 around islands with rats). P. lacrymatus is a herbivorous farmer damselfish that aggressively defends small solitary territories30, allowing territorial behaviour to be accurately linked to an individual’s nutritional resources.
Nutritional resources
Seabird guano contains high levels of the 15N nitrogen isotope relative to 14N (δ15N). Elevated levels of δ15N act as an indicator of seabird-derived nutrient subsidies and have also been positively associated with reef fish growth rate22, suggesting that δ15N is also an indicator of resource quality. Within P. lacrymatus territories adjacent to islands with seabirds, there was a 0.83 posterior probability (PP) that turf algal δ15N was higher than around islands with rats (Fig. 1; slope: 0.53, 95% credible intervals: −0.46, 1.58, evidence ratio (ER): 4.80). The posterior probability for this effect is similar to previous estimates of elevated turf algal δ15N adjacent to islands with seabirds22,25. Given the absence of any additional human stressors around the study islands, this isotopic enrichment can be attributed directly to seabird nutrient subsidies in the absence of invasive rats.
a,c, Each point on the violin plots represents a single P. lacrymatus territory. Black bars show the mean estimates for turf algal δ15N (a, n = 27 around islands with seabirds, n = 29 around islands with rats) and for turf algal cover (c, n = 20 around islands with seabirds, n = 30 around islands with rats). Means ± s.d. are presented above each violin plot. The upper and lower bounds of the violin plots show the range of the raw data. b,d, Bayesian posterior densities show the effect of island invasion status on turf algal δ15N (b) and turf algal cover (d). Points are median estimates, with thick and thin lines representing 75% and 95% credible intervals, respectively. PPs, ERs and posterior densities in green show the extent to which nitrogen input (b) and turf algal cover (d) are higher around islands with seabirds. Rat and seabird graphics from PhyloPic.org under Public Domain Dedication 1.0 licences.
There was no evidence for a difference in turf algal cover with island invasion status (slope: 0.02 (−0.13, 0.09), PP: 0.37, ER: 0.59; Fig. 1). While the absence of invasive rats does not appear to influence the amount of turf algae available to P. lacrymatus individuals, the nutritional enrichment of algae was higher within P. lacrymatus territories where seabird nutrient subsidies were present. Consequently, in the absence of invasive rats, P. lacrymatus achieve greater nutritional gain per unit of foraging effort.
Territory size
The greater nutritional gain per unit foraging effort around islands with seabirds resulted in P. lacrymatus individuals holding smaller territories compared with individuals around islands with rats, with a posterior probability of 0.99 (Fig. 2; slope: −0.21 (−0.34, −0.07), ER: 74.47). The nutritional content of algal resources can shape a trade-off between territory size and quality, with smaller territories yielding higher-quality nutritional resources31. Where nutrient subsidies are present, individuals can meet short-term energetic demands faster through a higher nutritional gain per unit foraging effort, allowing individuals to hold smaller territories7,32. Where seabird nutrient subsidies are absent, P. lacrymatus individuals need to consume a greater amount of turf algae to maintain short-term energetic demands. As there was no difference in the amount of turf algae available across the study islands, these individuals need to hold larger territories to maximize nutritional gain12 (Fig. 2). This observation is also reflected in the association between turf algal cover and turf algal δ15N around islands with rats, such that territories with the highest turf algal cover had the lowest δ15N values (Extended Data Fig. 2 and Table 1). There are, therefore, broad-scale differences in P. lacrymatus territory size that, given the lack of variation in turf algal cover with invasion status, are most likely driven by the observed differences in turf algal δ15N, which is a direct consequence of the presence or absence of invasive rats.
a, Raw data showing territory size estimates for P. lacrymatus individuals (n = 30 around islands with seabirds, n = 30 around islands with rats). Each point represents a single P. lacrymatus territory. Black bars show the mean estimates for P. lacrymatus territory size, and mean ± s.d. are also presented above each violin plot. The upper and lower bounds of the violin plots show the range of the raw data. b, Bayesian posterior density showing the effect of island invasion status on P. lacrymatus territory size. Points are median estimates, with thick and thin lines representing 75% and 95% credible intervals, respectively. The PP, ER and posterior density in green show the extent to which P. lacrymatus territories are smaller around islands with seabirds. c,e, Relationships between turf algal cover (e), turf algal δ15N (c) and P. lacrymatus territory size within island invasion status type. Points are partialized residuals extracted from Bayesian models for each P. lacrymatus individual. Best fit lines are extracted from Bayesian model conditional effects, with grey shading indicating 75% quantiles around the mean estimate. d,f, Posterior density plots showing the strength of the relationships in c and e, respectively. Densities to the right of 0 indicate a positive relationship, while densities to the left of 0 indicate a negative relationship. Evidence ratios show how much more likely the observed relationship is present over the alternative (grey shading). Rat and seabird graphics from PhyloPic.org under Public Domain Dedication 1.0 licences.
The design of our study allows the relative roles of resource quantity and quality in driving territoriality within and between island invasion status types to be understood. Nutritional resources are predicted to influence territoriality until a point of ‘consumer saturation’, at which point the benefits of territoriality have been maximized19. P. lacrymatus territory size was inversely associated with turf algal cover, providing support for the food maintenance hypothesis7,32 with a PP of 0.85 for islands with seabirds and 0.91 for islands with rats (Fig. 2; slope: −0.50 (−1.31, 0.32), ER: 5.70 around islands with seabirds, slope: −0.71 (−1.59, 0.17), ER: 10.24 around islands with rats). Around islands with seabirds, δ15N appeared to be at a point of consumer saturation19 such that the energetic benefits of elevated δ15N in determining territory size were maximized and fine-scale variation in δ15N had no further effect on P. lacrymatus territory size (slope: −0.01 (−0.09, 0.07), PP: 0.59, ER: 1.44; Fig. 2). There was also no association between P. lacrymatus territory size and turf algal δ15N around islands with rats (slope: 0.01 (−0.06, 0.08), PP: 0.38, ER: 0.60). In the absence of nutrient subsidies where overall turf algal δ15N is low, slight variation in turf algal δ15N probably yields a lower energetic pay-off18,19 than variation in turf algal cover, indicating a trade-off between resource quality and quantity. As the evidence for both smaller territory sizes (PP = 0.99) and decreasing territory size with increasing turf algal cover ratio (PP = 0.91) for P. lacrymatus around islands with rats is relatively strong, resource quantity appears to be the primary determinant of P. lacrymatus territory size where nutrient subsidies are absent. These results offer strong support that nutrient disruption by an invasive terrestrial species has direct consequences on the territoriality of a native marine species.
Aggression
The higher nutritional gain per unit foraging effort available to P. lacrymatus adjacent to islands with seabirds provides individuals with the energy to invest more time in aggressive territory defence than individuals adjacent to islands with invasive rats, with a PP of 0.85 (Fig. 3; slope: 0.47 (−0.35, 1.28), ER = 5.47)9,33. Furthermore, this broad-scale effect is driven by fine-scale variation in turf algal cover and δ15N in P. lacrymatus territories within island invasion status. Adjacent to islands with seabirds, the overall elevated turf algal δ15N has maximized the benefits of turf algal δ15N for P. lacrymatus aggression, resulting in no relationship between fine-scale variation in δ15N and aggression (Fig. 3; slope: −0.11 (−0.43, 0.21), PP: 0.29, ER: 0.41). Variation in aggression is instead driven by turf algal cover within each P. lacrymatus individual’s territory (Fig. 3; slope: 2.97 (−0.72, 6.43), PP: 0.91, ER: 10.15). As turf algal cover increases, the value of the territory increases, and the pay-off of being aggressive is higher than remaining passive in the presence of nutrient subsidies19,34. It is also possible that variation in aggression is a consequence of P. lacrymatus having evolved an inherently lower threshold for aggression in the absence of invasive rats. Aggression is heritable35, and the invasion status of the study islands has been consistent since the introduction of black rats in the 1700s36.The short larval duration of P. lacrymatus (~23 days)37 and the prevalence of self-recruitment among reef fish38 suggest it is plausible that P. lacrymatus individuals adjacent to islands with seabirds only recruit to islands with seabirds and vice-versa. Both the evolutionary history of the economics of territoriality and the behavioural history of individuals39 can have important consequences for the persistence of species11.
a, Raw aggression estimates for P. lacrymatus individuals (n = 28 around islands with seabirds, n = 29 around islands with rats). Each point represents a single P. lacrymatus territory. Black bars are mean estimates, and mean ± s.d. are also presented above each violin plot. The upper and lower bounds of the violin plots show the range of the raw data. b, Bayesian posterior density showing the effect of island invasion status on P. lacrymatus aggression. Points are median estimates, with lines representing 75% and 95% credible intervals, respectively. The PP, ER and posterior density in green show the extent to which P. lacrymatus aggression is higher around islands with seabirds. c,e, Relationships between turf algal cover (e), turf algal δ15N (c) and P. lacrymatus aggression within island invasion status type. Points are partialized residuals extracted from Bayesian models, with best fit lines extracted from Bayesian model conditional effects. Grey shading indicates 75% quantiles around the mean estimate. d,f, Posterior density plots showing the strength of the relationships in c and e, respectively. Densities to the right of 0 indicate a positive relationship, while densities to the left of 0 indicate a negative relationship. Evidence ratios show how much more likely the observed relationship is present over the alternative (grey shading). Rat and seabird graphics from PhyloPic.org under Public Domain Dedication 1.0 licences.
In the presence of invasive rats and the subsequent absence of nutrient subsidies, the costs of aggression (energetic expenditure) appear to outweigh the benefits (energetic gain) even where turf algal cover is high. The resource value of these P. lacrymatus territories primarily remains below the threshold where aggression would be beneficial, resulting in no association between either turf algal cover (slope: 0.00 (−3.48, 3.45), PP: 0.50, ER: 1.02) or δ15N (slope: 0.09 (−0.26, 0.45), PP: 0.66, ER: 1.98; Figs. 3 and 4). The disruption of nutrients by invasive black rats has therefore reduced the aggressive tendencies of P. lacrymatus individuals (Fig. 4).
Territoriality is predicted to occur where the benefits outweigh the cost (shaded blue area). Below the threshold of territoriality, there is predicted to be no relationship between resource value and territoriality (dashed red boxes). The presence of nutrient subsidies around islands with seabirds is predicted to increase resource value to damselfish, resulting in higher levels of aggression (green point) than around islands with invasive rats (orange point). An inverse relationship between resource value and territory size (secondary y axis) is also predicted such that territories of higher resource value, that is, those around islands with seabirds, will be smaller (circular bird icon) than territories with lower resource value, that is, around islands with invasive rats (circular rat icon). Around islands with rats, resource value is low, and variation in turf algal cover and turf algal δ15N is not enough to place P. lacrymatus individuals above the threshold of territoriality (orange arrows). Around islands with seabirds, elevated δ15N is high, placing P. lacrymatus territories beyond the threshold of territoriality (green arrow with open arrowhead). Variation in aggression within reefs adjacent to islands with seabirds is instead driven by variation in turf algal cover (green arrow with closed arrowhead). Rat and seabird graphics from PhyloPic.org under Public Domain Dedication 1.0 licences.
As farming damselfish, P. lacrymatus have the capacity to add nutrients to their algal farm, resulting in a higher productivity of algal turfs within P. lacrymatus territories compared with algal turfs outside the territories40. Around rat-infested islands, it is therefore possible that lower aggression by P. lacrymatus was traded off by an increase in farming behaviours to raise the productivity of algal resources within P. lacrymatus territories. However, as the focus of this study is on the specific role of seabird-derived nutrients, i.e. δ15N within algal resources, the impact of P. lacrymatus adding nutrients to their algal farms on our results and inferences is likely to be negligible. Similarly, seasonal variation in nutrient cycling that may impact the seabird-derived nutrient loads onto the coral reefs around our study sites may also be negligible. We controlled for environmental variation that could impact nutrient loads, such as weather conditions (see Methods). Furthermore, as there are nesting seabirds on rat-free islands year-round, including species that are large contributors to the seabird-derived nutrient budget41, seasonal variation in seabird densities is also unlikely to have a substantial impact on the observed differences in P. lacrymatus behaviour in the presence and absence of seabird-derived nutritional subsidies.
Ecosystem-level consequences
The impact of invasive species in terrestrial ecosystems on the territoriality of reef fish (Fig. 4, Extended Data Fig. 3, and Supplementary results and discussion) could have broader community-level consequences for coral reef ecosystems. P. lacrymatus individuals adjacent to rat-infested islands display lower growth rates than individuals with access to seabird nutrient subsidies22. This has important consequences for fish biomass and productivity, with faster-growing, more aggressive fish adjacent to rat-free islands probably contributing to increased ecosystem function in the presence of seabird nutrient subsidies22,29.
As a functionally important reef fish42, the aggressive nature of P. lacrymatus and other territorial damselfish can influence the spatial and social organization of reef fish communities. For example, space use and foraging areas of butterflyfish (Chaetodontidae) are influenced by high levels of interspecific aggression from damselfish43. The density of surgeonfishes, such as Acanthurus coeruleus, is also negatively associated with territorial damselfish density, and damselfish density is a predictor of surgeonfish social mode44. Furthermore, by driving variation in P. lacrymatus territory size, the disruption of nutrients by invasive rats also affects the spatial organization of territorial damselfish (Extended Data Figs. 4–6 and Table 2), which may have broader impacts on the reef community. P. lacrymatus have the capacity to influence the composition of both algal and coral communities45. Damselfish territories are areas of high algal productivity46, but also act as a refuge for some species of coral46 while contributing to the mortality of others47. The role of invasive rats in altering P. lacrymatus territory size could therefore have indirect consequences for coral growth, community composition and resilience.
We have provided new insights into how the disruption of cross-ecosystem nutrient subsidies by a terrestrial invasive species can influence territorial behaviour in a reef fish. Given the global decline in the movement of nutrients across ecosystems, understanding the proximate ecological implications of nutritional declines, such as behavioural responses of affected organisms, is a fundamental step in understanding the ultimate consequences of declining nutrient subsidies on species persistence11. The presence of invasive rats impacts reef fish species interactions via changes in territorial behaviour, impacting benthic and reef fish community composition and biodiversity, and subsequently ecosystem function and resilience25,29. Rat eradication therefore has the potential to have multiple cross-ecosystem benefits, from restoring territoriality in individual reef fish to the subsequent bottom-up effects on populations, communities and ecosystems.
Methods
Site and study species
We completed all data collection between 14 April and 6 May 2021 around the remote northern islands of the Chagos archipelago, which is part of a large no-take marine protected area in the Indian Ocean48. The northern reefs of the archipelago are some of the most pristine in the world, characterized by extremely high fish biomass22. The study area is free of local human stressors, except for invasive rats36. In total we surveyed 10 islands across 3 atolls (Extended Data Fig. 1). Five of the islands are infested with black rats (Rattus rattus) and 5 have high seabird densities and no rat infestations. Seabird densities around rat-free islands have been shown to be 760 times higher than around rat-infested islands22. Otherwise, all islands are similar in terms of environment and size36,48. Surveys addressing the impact of seabird-derived nutrient subsidies on both terrestrial and marine ecosystems have been conducted around all of the study islands every year between March and April since 2015. All work was conducted on the inward side of the islands close to shore. Survey sites were therefore selected to reduce the impact of variables such as seasonal variation in water and weather conditions.
Plectroglyphidodon lacrymatus is categorized as an extensive herbivorous farmer, with both macroalgae and turf algae found within their algal farms30. P. lacrymatus has also been used in previous studies to quantify nitrogen isotope signals in the presence and absence of invasive rats across the Chagos Archipelago22. Specifically, P. lacrymatus around rat-free islands have also been shown to have an elevated nitrogen signature (δ15N) and faster growth rate compared with individuals around rat-infested islands22.
Behavioural observations
At each of the 10 islands, we randomly selected 6 focal P. lacrymatus individuals from around sites that were surveyed in 201522 and in 201827. These survey sites were marked by a GPS in 2015, and the distance of these sites from the island shore was also recorded22. We placed a Go Pro camera in the vicinity of the territories of the focal P. lacrymatus individuals. Cameras were deployed for 20 min, with the first 5 min discarded as an acclimation period49 to ensure the focal individual was not influenced by the presence of observers or the camera. After 20 min, cameras were collected and videos analysed in the laboratory. We used a continuous sampling approach when conducting video analyses, recording behaviours and the time at which behaviour changed. Videos were recorded between approximately 9 am and 3 pm. While there is evidence that P. lacrymatus foraging behaviour varies with the time of day50, we considered population-level behaviour in our analyses, and recording videos across a 6 h time period allowed us to capture this natural variation. Subsequently, we were able to determine whether differences in aggressive behaviour are present as a consequence of the presence/absence of nutritional subsidies while controlling for natural variation in foraging behaviour, which could in turn result in natural variation in aggressive tendencies.
We recorded all P. lacrymatus aggressive interactions from the behavioural videos using the BORIS software51. Behaviours associated with aggressive interactions included attacking in short accelerated swimming movements, biting and butting52. For all aggressive interactions, the encountered species was recorded as either a conspecific or heterospecific. We noted both the number of aggressive interactions and the total length of all aggressive interactions for each individual. We were unable to determine the sex of focal individuals as there is no sexual dimorphism for this species, and we did not collect the focal individuals to determine sex via dissection. While it is possible that there could be an effect of sex on territorial behaviour, this would probably only be the case for territoriality associated with mating, specifically egg guarding, and there was no evidence of such behaviour in any of our videos. All behavioural videos are available via Figshare (https://doi.org/10.6084/m9.figshare.21089770).
Territory mapping
We mapped the territory of a single individual present at the location of each of the six Go Pro cameras placed for behavioural studies at each island using methods similar to those in ref. 30. We placed a camera on a stand 1 m above the territory of the individual such that the camera had a field of view of 2 × 2 m. A 25 cm scale was visible within the field of view of the camera. Cameras were left for 20 min, with the first 9 min then discarded as an acclimation period49. To calculate territory size, we took 21 screengrabs of the footage approximately every 30 s across a 10 min period. We then imported these screengrabs into ImageJ to record the position of the focal individual. We set the frame of the images as X, Y axes and recorded the individual positions as Cartesian coordinates. We then plotted the Cartesian coordinate positions and calculated the minimum convex polygon of the points for each focal damselfish to estimate territory area.
We also used the territory camera footage to count the number of neighbouring P. lacrymatus individuals to estimate P. lacrymatus density for each individual patch surveyed within each island. The total length of each focal individual was also estimated from the territory camera footage.
For two of the islands (one rat-invested island and one island without rats, and a total of 12 P. lacrymatus individuals), territory cameras could not be used due to high currents and wave surge. Instead, we used Go Pro cameras on a photo time-lapse (one photo per second) to take photos of the focal damselfish territory, with a 0.5 × 0.5 m quadrat placed within the territory as a size reference. The photos were then imported into the software programme Agisoft and used to create three-dimensional (3D) models of the focal individual territories. To estimate territory size for these individuals, we used the behavioural observation video to mark the location of focal individuals every 30 s for 10 min as above, then cross referenced these locations with a screengrab of the 3D model in ImageJ using the frame of the image as X, Y axes as above. To estimate the total length of these individuals, we photographed each territory from above, with a 0.5 × 0.5 m quadrat as a size reference, ensuring the focal individual was also within the frame of the photo. These images were then imported into ImageJ and the size of each focal individual was estimated.
Benthic composition
Benthic surveys were conducted using the territory mapping video footage. We took a screengrab of the territory area from the videos and overlaid 100 points onto this image. We recorded the benthos under each of these points to quantify the abundance and percentage cover of algae (turf and macro) within each territory. For the 12 individuals for which territory cameras could not be used, we utilized the 3D models to conduct the territory benthic surveys using the same methods as above.
Isotope sampling
Following behavioural observations and territory mapping, we collected turf algae and macroalgae if present (Halimeda spp.) from within the territory of each focal individual. All algal samples were dried at 60 °C for 24 h or until dry, ahead of nitrogen stable isotope analysis. Dried samples were washed in a 10% hydrochloric acid solution to remove any contaminants and calcareous matter, and then centrifuged at 1,008 × g (3,000 r.p.m.) for 6 min. Samples were washed thoroughly with distilled water between centrifuge cycles. In total, samples were centrifuged four times. Samples were then dried for a final time. Isotope samples were then analysed at Lancaster University (UK). Samples were combusted using an Elementar Vario MICRO cube elemental analyser before being analysed in an Isoprime 100 isotope ratio mass spectrometer. Samples were analysed with the two international standards IAEA 600 and USGS 41, and a random subset of samples were run in triplicates to ensure readings were accurate. From the analysis, we extracted values for the ratios of the nitrogen isotope N15:14 (δ15N) for both turf and macroalgae.
Statistical analysis
All our analyses were conducted using R v4.1.053. We ran Bayesian models using the brms package54 implemented in STAN55. All Bayesian models were run for 5,000 iterations, with a warm-up of 1,000 iterations over four chains. We used weakly informative normal priors for all models56 and included a nested random intercept for atoll and the islands within each atoll to account for spatial non-independence. As there was no ecological basis to assume variation in slopes between the different islands within each atoll, we did not include random slopes in the models22,27. All models assumed a normal likelihood based on distribution plots for each response variable. To check model fit and convergence, we used trace-plots, graphical posterior predictive checks, effective sample sizes and the Gelman-Ruban convergence diagnostic (R-hat)57. We log-transformed the time invested in aggression to improve model convergence and remove divergent transitions that were present when we ran the model on untransformed data. A total of 5 models had up to 10 divergent transitions. However, all models had effective sample size values of over 1,000 and R-hat values of less than 1.01, indicating that the Markov) chains had converged well58,59. To check for heavily weighted influential data points in our models, we used Pareto-smoothed importance-sampling leave-one-out cross-validation (PSIS_LOO). Pareto-k values of over 0.7 are considered highly influential59. Where we had pareto-k values of over 0.7, we ran the models with and without the highly influential data points and compared the posterior distributions. We also extracted the conditional effects and partialized residuals from all models. We then used the conditional effects and partialized residuals to determine mean estimates and 75% uncertainty intervals of the posterior predictive distributions. All our models comparing between island invasion status therefore had the following basic structure:
Our models comparing aspects of nutritional resources and P. lacrymatus territoriality within each island invasion status type had the following basic structure:
We then used hypothesis testing to test a priori hypotheses for our models. All hypothesis tests were one-way, and for each test we calculated: the PP to determine the probability to which our hypotheses were supported, and ER to show the extent to which the evidence that our hypotheses is supported is greater than an alternative hypothesis (Extended Data Tables 1 and 2). Evidence ratios are transformed posterior probability values such that \(\mathrm{ER} = \frac{{\mathrm{PP}}}{{(1 - \mathrm{PP})}}\).
Nutritional resources
We compared turf algal δ15N within focal individual territories using a Bayesian model, with island invasion status as the explanatory variable. The following hypothesis was then tested, and posterior probabilities and evidence ratios calculated using nonlinear hypothesis testing: δ15N will be higher for P. lacrymatus territories around islands where rats are absent.
To compare the proportion of turf algae within focal individual territories between islands with no rats and islands with rats, we used a Bayesian model with island invasion status (rats absent/rats present) as the explanatory variable. We then used a nonlinear hypothesis test to test the a priori hypothesis that there is a higher proportion of turf algae around rat-infested islands.
We also ran a model to test for a relationship between resource quality (turf algal δ15N) and quantity (turf algal cover) within P. lacrymatus territories, with δ15N as the response variable. We used an interaction term between turf algal cover and island invasion status, as the relationship between resource quality and quantity could be variable between islands with seabirds and islands with rats. We then used nonlinear hypothesis tests to test the strength of the relationship between turf algal δ15N and cover around islands with seabirds and islands with rats, and to see whether the relationship was different between the two island types.
To see whether turf algal δ15N and cover were influenced by the distance of the territory to shore, we ran two models, with an interaction term between the distances from the GPS marked survey sites to the shore and invasion status, and turf algal δ15N or cover as the response variable. We then tested the following a priori hypothesis for both models: Turf algal δ15N/cover will be higher around sites closest to shore for islands with seabirds.
Territory size and aggression between island types
We tested the effect of island invasion status on P. lacrymatus territory size, time spent on aggression, conspecific density and focal individual total length using Bayesian models, with island invasion status (rats absent/rats present) as the explanatory variable. The length of aggressive interactions was log-transformed to improve model fit. We performed hypothesis tests for four a priori hypotheses:
-
1.
P. lacrymatus territory sizes will be smaller around islands where rats are absent.
-
2.
P. lacrymatus aggression (in terms of the length of aggressive interactions) will be higher around islands where rats are absent.
-
3.
P. lacrymatus densities will be higher around islands where rats are absent.
-
4.
P. lacrymatus total length will be higher around islands where rats are absent.
We ran a Bayesian model to test the relative roles of resource quality and quantity on P. lacrymatus territory size. We ran two models, one with resource quantity (turf algal cover) as the explanatory variable, and the other with resource quality (turf algal δ15N) as the explanatory variable. We included an interaction term between the explanatory variable and island invasion status. We then tested two a priori hypotheses from each of the two models: Around islands with seabirds (1) and islands with rats (2), territories with a higher turf algal δ15N/cover will be smaller.
Territory size and aggression within island type
For both aggressive behaviour and territory size, we considered the influence of turf algal δ15N, turf algal cover, conspecific density and focal individual total length within islands with seabirds and islands with rats, including an interaction term between each explanatory variable and island invasion status. We considered multiple variables to determine the strength of the relationship between territoriality and nutritional resources while controlling for additional biotic variables (conspecific density and total length). We ran two separate models for territory size and aggression, with all four of the above explanatory variables included in each model. We then ran a priori hypothesis tests to determine the strength of relationships between territory size and aggressive behaviour and each of the four explanatory variables.
In addition, we looked at the influence of territory size on aggression with an additional Bayesian model, with aggression as the response variable and territory size as the explanatory variable, with an interaction term between the explanatory variable and island invasion status also included. We used nonlinear hypothesis tests on this model to test the following a priori hypotheses: Around islands with seabirds (1) and islands with rats (2), aggression will be highest for P. lacrymatus individuals in the smallest territories, and this relationship will be weaker around islands with rats (3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The dataset associated with this work will made publicly available through the Figshare repository at https://doi.org/10.6084/m9.figshare.21089770
Code availability
The code associated with this work will be made publicly available through the Figshare repository at https://doi.org/10.6084/m9.figshare.21089770
References
Polis, G. A., Anderson, W. B. & Holt, R. D. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Evol. Syst. 28, 289–316 (1997).
Doughty, C. E. et al. Global nutrient transport in a world of giants. Proc. Natl Acad. Sci. USA 113, 868–873 (2016).
Burpee, B. T. & Saros, J. E. Cross-ecosystem nutrient subsidies in Arctic and alpine lakes: implications of global change for remote lakes. Environ. Sci. 22, 1166–1189 (2020).
Gallardo, B., Clavero, M., Sánchez, M. I. & Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Change Biol. 22, 151–163 (2016).
Justino, D. G., Maruyama, P. K. & Oliveira, P. E. Floral resource availability and hummingbird territorial behaviour on a Neotropical savanna shrub. J. Ornithol. 153, 189–197 (2012).
Van Overveld, T. et al. Food predictability and social status drive individual resource specializations in a territorial vulture. Sci. Rep. 8, 15155 (2018).
Gunn, R. L., Hartley, I. R., Algar, A. C., Nadiarti, N. & Keith, S. A. Variation in the behaviour of an obligate corallivore is influenced by resource availability. Behav. Ecol. Sociobiol. https://doi.org/10.1007/s00265-022-03132-6 (2022).
Keith, S. A. et al. Synchronous behavioural shifts in reef fishes linked to mass coral bleaching. Nat. Clim. Change 8, 986–991 (2018).
Davies, N. B. & Hartley, I. R. Food patchiness, territory overlap and social systems: an experiment with dunnocks Prunella modularis. J. Anim. Ecol. 65, 837–846 (1996).
Cahill, A. E. et al. How does climate change cause extinction? Proc. R. Soc. B 280, 20121890 (2013).
Delarue, P. E. M., Kerr, S. E. & Rymer, T. L. Habitat complexity, environmental change and personality: a tropical perspective. Behav. Process. 120, 101–110 (2015).
Stimson, J. The role of the territory in the ecology of the intertidal limpet Lottia gigantea (Gray). Ecology 54, 1020–1030 (1973).
Sells, S. N. & Mitchell, M. S. The economics of territory selection. Ecol. Modell. 438, 109329 (2020).
Graf, P. M., Mayer, M., Zedrosser, A., Hackländer, K. & Rosell, F. Territory size and age explain movement patterns in the Eurasian beaver. Mamm. Biol. 81, 587–594 (2016).
Simon, C. The influence of food abundance on territory size in the Iguanid lizard Sceloporus jarrovi. Ecology 56, 993–998 (1975).
Ippi, S., Cerón, G., Alvarez, L. M., Aráoz, R. & Blendinger, P. G. Relationships among territory size, body size, and food availability in a specialist river duck. Emu 118, 293–303 (2018).
Berumen, M. L. & Pratchett, M. S. Effects of resource availability on the competitive behaviour of butterflyfishes (Chaetodontidae). In Proc. 10th International Coral Reef Symposium 644–650 (ReefBase, 2006); http://reefbase.org/resource_center/publication/icrs.aspx?icrs=ICRS10
Brown, J. L. The evolution of diversity in avian territorial systems. Wilson Bull. 76, 160–169 (1964).
Peiman, K. S. & Robinson, B. W. Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol. 85, 133–158 (2010).
Grant, J. W. A., Girard, I. L., Breau, C. & Weir, L. K. Influence of food abundance on competitive aggression in juvenile convict cichlids. Anim. Behav. 63, 323–330 (2002).
Duda, M. P. et al. Long-term changes in terrestrial vegetation linked to shifts in a colonial seabird population. Ecosystems 23, 1643–1656 (2020).
Graham, N. A. J. et al. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559, 250–253 (2018).
Jones, H. P. et al. Severity of the effects of invasive rats on seabirds: a global review. Conserv. Biol. 22, 16–26 (2008).
Honig, S. E. & Mahoney, B. Evidence of seabird guano enrichment on a coral reef in Oahu, Hawaii. Mar. Biol. 163, 22 (2016).
Benkwitt, C. E., Gunn, R. L., Le Corre, M., Carr, P. & Graham, N. A. J. Rat eradication restores nutrient subsidies from seabirds across terrestrial and marine ecosystems. Curr. Biol. 31, 2704–2711.e4 (2021).
Savage, C. Seabird nutrients are assimilated by corals and enhance coral growth rates. Sci. Rep. 9, 4284 (2019).
Benkwitt, C. E., Wilson, S. K. & Graham, N. A. J. Seabird nutrient subsidies alter patterns of algal abundance and fish biomass on coral reefs following a bleaching event. Glob. Change Biol. 25, 2619–2632 (2019).
Benkwitt, C. E., Taylor, B. M., Meekan, M. G. & Graham, N. A. J. Natural nutrient subsidies alter demographic rates in a functionally important coral-reef fish. Sci. Rep. 11, 12575 (2021).
Benkwitt, C. E., Wilson, S. K. & Graham, N. A. J. Biodiversity increases ecosystem functions despite multiple stressors on coral reefs. Nat. Ecol. Evol. 4, 919–926 (2020).
Robles, H. & Martin, K. Resource quantity and quality determine the inter-specific associations between ecosystem engineers and resource users in a cavity-nest web. PLoS ONE 8, e74694 (2013).
Catano, L. B., Gunn, B. K., Kelley, M. C. & Burkepile, D. E. Predation risk, resource quality, and reef structural complexity shape territoriality in a coral reef herbivore. PLoS ONE 10, e0118764 (2015).
Wilcox, K. A., Wagner, M. A. & Reynolds, J. D. Salmon subsidies predict territory size and habitat selection of an avian insectivore. PLoS ONE 16, e0254314 (2021).
Frost, S. K. & Frost, P. G. H. Territoriality and changes in resource use by sunbirds at Leonotis leonurus (Labiatae). Oecologia 45, 109–116 (1980).
Maynard Smith, J. Evolution and the Theory of Games (Cambridge Univ. Press, 1982).
Dochtermann, N. A., Schwab, T., Anderson Berdal, M., Dalos, J. & Royauté, R. The heritability of behavior: a meta-analysis. J. Hered. 110, 403–410 (2019).
Sheppard, C. R. C. et al. Reefs and islands of the Chagos Archipelago, Indian Ocean: why it is the world’s largest no-take marine protected area. Aquat. Conserv. 22, 232–261 (2012).
Soeparno, Y. N., Shibuno, T. & Yamaoka, K. Relationship between pelagic larval duration and abundance of tropical fishes on temperate coasts of Japan. J. Fish. Biol. 80, 346–357 (2012).
Green, A. L. et al. Larval dispersal and movement patterns of coral reef fishes, and implications for marine reserve network design. Biol. Rev. 90, 1215–1247 (2015).
Dall, S. R. X., Houston, A. I. & McNamara, J. M. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 (2004).
Klumpp, D., McKinnon, D. & Daniel, P. Damselfish territories: zones of high productivity on coral reefs. Mar. Ecol. Prog. Ser. 40, 41–51 (1987).
Carr, P. et al. Status and phenology of breeding seabirds and a review of important bird and biodiversity areas in the British Indian Ocean Territory. Bird Conserv. Int. 31, 14–34 (2020).
Hoey, A. S. & Bellwood, D. R. Damselfish territories as a refuge for macroalgae on coral reefs. Coral Reefs 29, 107–118 (2010).
Samways, M. J. Breakdown of butterflyfish (Chaetodontidae) territories associated with the onset of a mass coral bleaching event. Aquat. Conserv. 15, 101–107 (2005).
Morgan, I. E. & Kramer, D. L. Determinants of social organization in a coral reef fish, the blue tang, Acanthurus coeruleus. Environ. Biol. Fishes 72, 443–453 (2005).
Ceccarelli, D. M. Modification of benthic communities by territorial damselfish: a multi-species comparison. Coral Reefs 26, 853–866 (2007).
Gochfeld, D. J. Territorial damselfishes facilitate survival of corals by providing an associational defense against predators. Mar. Ecol. Prog. Ser. 398, 137–148 (2010).
Gordon, T. A. C., Cowburn, B. & Sluka, R. D. Defended territories of an aggressive damselfish contain lower juvenile coral density than adjacent non-defended areas on Kenyan lagoon patch reefs. Coral Reefs 34, 13–16 (2015).
Hays, G. C. et al. A review of a decade of lessons from one of the world’s largest MPAs: conservation gains and key challenges. Mar. Biol. 167, 159–167 (2020).
Nanninga, G. B., Côté, I. M., Beldade, R. & Mills, S. C. Behavioural acclimation to cameras and observers in coral reef fishes. Ethology 123, 705–711 (2017).
Polunin, N. V. C. & Klumpp, D. W. Ecological correlates of foraging periodicity in herbivorous reef fishes of the Coral Sea. J. Exp. Mar. Biol. Ecol. 126, 1–20 (1989).
Friard, O. & Gamba, M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330 (2016).
Paola, V. D., Vullioud, P., Demarta, L., Alwany, M. A. & Ros, A. F. H. Factors affecting interspecific aggression in a year-round territorial species, the jewel damselfish. Ethology 118, 721–732 (2012).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Bürkner, P. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Soft. 80, 1–28 (2017).
RStan: the R interface to Stan. R package version 2.21.5 (Stan Development Team, 2022).
Hadfield, J. D. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Soft. 33, 1–22 (2010).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Vehtari, A., Gelman, A., Simpson, D., Carpenter, B. & Burkner, P. C. Rank-normalization, folding, and localization: an improved (formula presented) for assessing convergence of MCMC (with Discussion). Bayesian Anal. 16, 667–718 (2021).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
Acknowledgements
Fieldwork was conducted under permit number 0002SE21. Funding was provided by the Bertarelli Foundation and contributed to the Bertarelli Programme in Marine Science. This work was also supported by the Natural Environment Research Council (grant number NE/S00050X/1 to S.A.K and grant number NE/L002604/1 for R.L.G), with R.L.G.’s studentship supported through the Envision Doctoral Training Partnership.
Author information
Authors and Affiliations
Contributions
R.L.G. conceived the ideas, collected the data, analysed the data and led the writing of the manuscript. C.E.B helped conceive the ideas, supported data collection and analysis, and contributed critically to manuscript drafts. N.A.J.G supported data analysis and contributed critically to manuscript drafts. I.R.H., A.C.A. and S.A.K. contributed critically to manuscript drafts. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Paul-Christian Bürkner and Josefin Sundin for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
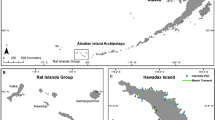
Extended Data Fig. 1 The location of study sites around the Chagos Archipelago.
The location of the Chagos Archipelago in the Indian ocean is shown in the inset. Surveys were conducted around three atolls: Peros Banhos (PB), Salomon (SAL) and the Great Chagos Bank (GCB). Points represent the locations of the 10 reefs where surveys were conducted.
Extended Data Fig. 2 The relationship between turf algal cover and δ15N within P. lacrymatus territories.
Top: Points represent partialized residuals extracted from Bayesian models for each P. lacrymatus individual around islands with seabirds (Green) and islands with rats (Yellow). Points are presented alongside best fit lines based on Bayesian model conditional effects, with grey shading indicating 75% quantiles. Bottom: Bayesian posterior densities from hypothesis tests. Posterior probabilities and evidence ratios show the extent to which 1) a positive relationship is supported around islands with seabirds (Left, green), 2) A negative relationship is supported around islands with rats (Middle, yellow), and 3) The relationship between turf algal cover and δ15N is different (that is, more negative) for P. lacrymatus territories around islands with rats compared to around islands with seabirds (Right, yellow).
Extended Data Fig. 3 The influence of P. lacrymatus territory size on aggression around islands with seabirds (Green) and islands with rats (Yellow).
Points represent partialized residuals extracted from Bayesian models for each P. lacrymatus. Best fit lines are extracted from Bayesian model conditional effects, with grey shading indicating 75% quantiles around the mean estimate.
Extended Data Fig. 4 P. lacrymatus density and focal individual total length around islands with seabirds and islands with invasive rats within the Chagos Archipelago.
Each point on the violin plots (left) represents a single P. lacrymatus territory. Mean estimates for conspecific density (A, left) around islands with seabirds (n = 30) and islands with rats (n = 30), and for focal individual total length (B, left) around islands with seabirds (n = 30) and islands with rats (n = 30) are represented by black bars. Posterior densities (Right) in green show the extent to which the following hypotheses are supported.: 1. Conspecific density (A, right) is higher around islands with seabirds and 2. Focal individual total length (B, right) is higher around islands with seabirds. Evidence ratios show how much more likely these hypotheses are supported over the alternative hypotheses. Rat and seabird graphics from PhyloPic.org under Public Domain Dedication 1.0 licenses.
Extended Data Fig. 5 P. lacrymatus conspecific density, nutritional resources, and P. lacrymatus territoriality.
Points represent partialized residuals extracted from Bayesian models for each P. lacrymatus individual around islands with seabirds (Green) and islands with rats (Yellow). Best fit lines are extracted from Bayesian model conditional effects, with grey shading indicating 75% quantiles 75% quantiles around the mean estimate.
Extended Data Fig. 6 P. lacrymatus total length, nutritional resources, and P. lacrymatus territoriality.
Points represent partialized residuals extracted from Bayesian models for each P. lacrymatus individual around islands with seabirds (Green) and islands with rats (Yellow). Best fit lines are extracted from Bayesian model conditional effects, with grey shading indicating 75% quantiles 75% quantiles around the mean estimate.
Supplementary information
Supplementary Information
Supplementary methods, results and discussion and extended data legends.
Supplementary Data
BORIS file containing the raw analysis of behavioural videos.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunn, R.L., Benkwitt, C.E., Graham, N.A.J. et al. Terrestrial invasive species alter marine vertebrate behaviour. Nat Ecol Evol 7, 82–91 (2023). https://doi.org/10.1038/s41559-022-01931-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01931-8
This article is cited by
-
Invasive species drive cross-ecosystem effects worldwide
Nature Ecology & Evolution (2024)
-
Seabird guano reshapes intertidal reef food web in an isolated oceanic islet
Coral Reefs (2024)
-
Predation of sea turtle eggs by rats and crabs
Marine Biology (2024)