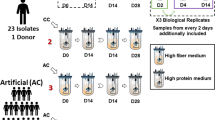
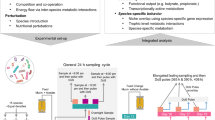
Abstract
Dietary fibre impacts the growth dynamics of human gut microbiota, yet we lack a detailed and quantitative understanding of how these nutrients shape microbial interaction networks and responses to perturbations. By building human gut communities coupled with computational modelling, we dissect the effects of fibres that vary in chemical complexity and each of their constituent sugars on community assembly and response to perturbations. We demonstrate that the degree of chemical complexity across different fibres limits microbial growth and the number of species that can utilize these nutrients. The prevalence of negative interspecies interactions is reduced in the presence of fibres compared with their constituent sugars. Carbohydrate chemical complexity enhances the reproducibility of community assembly and resistance of the community to invasion. We demonstrate that maximizing or minimizing carbohydrate competition between resident and invader species enhances resistance to invasion. In sum, the quantitative effects of carbohydrate chemical complexity on microbial interaction networks could be exploited to inform dietary and bacterial interventions to modulate community resistance to perturbations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Additional data supporting the findings described in this work are available from the corresponding author on reasonable request. The processed data for all community experiments and simulations are available in GitHub (https://github.com/VenturelliLab/Ostrem_Loss_et_al_2022) and in the source data provided with this paper. The Illumina sequencing data are available via Zenodo at https://zenodo.org/record/7067625#.Yx1COXbMJPY.
Code availability
Code for model training and simulations is available in GitHub (https://github.com/VenturelliLab/Ostrem_Loss_et_al_2022).
References
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Schmidt, T. S. B., Raes, J. & Bork, P. The human gut microbiome: from association to modulation. Cell 172, 1198–1215 (2018).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Tap, J. et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 17, 4954–4964 (2015).
Smits, S. A. et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 357, 802–806 (2017).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Morrison, K. E., Jašarević, E., Howard, C. D. & Bale, T. L. It’s the fiber, not the fat: significant effects of dietary challenge on the gut microbiome. Microbiome 8, 15 (2020).
Maslowski, K. M. & Mackay, C. R. Diet, gut microbiota and immune responses. Nat. Immunol. 12, 5–9 (2011).
Reynolds, A. et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 393, 434–445 (2019).
Slavin, J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435 (2013).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353.e21 (2016).
Makki, K., Deehan, E. C., Walter, J. & Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018).
Cantu-Jungles, T. M. et al. Dietary fiber hierarchical specificity: the missing link for predictable and strong shifts in gut bacterial communities. mBio 12, e01028-21 (2022).
Murga-Garrido, S. M. et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 9, 117 (2021).
Cantu-Jungles, T. M. & Hamaker, B. R. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio 11, e02179-19 (2020).
Singh, V. et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 175, 679–694.e22 (2018).
Terrapon, N., Lombard, V., Gilbert, H. J. & Henrissat, B. Automatic prediction of polysaccharide utilization loci in Bacteroidetes species. Bioinformatics 31, 647–655 (2015).
Terrapon, N. et al. PULDB: the expanded database of Polysaccharide Utilization Loci. Nucleic Acids Res. 46, D677–D683 (2018).
Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M. & Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 (2014).
Kouzuma, A., Kato, S. & Watanabe, K. Microbial interspecies interactions: recent findings in syntrophic consortia. Front. Microbiol. 6, 477 (2015).
Faust, K. & Raes, J. Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550 (2012).
Rakoff-Nahoum, S., Coyne, M. J. & Comstock, L. E. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr. Biol. 24, 40–49 (2014).
Luis, A. S. et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 3, 210–219 (2018).
Cartmell, A. et al. A surface endogalactanase in Bacteroides thetaiotaomicron confers keystone status for arabinogalactan degradation. Nat. Microbiol. 3, 1314–1326 (2018).
Rakoff-Nahoum, S., Foster, K. R. & Comstock, L. E. The evolution of cooperation within the gut microbiota. Nature 533, 255–259 (2016).
Pichler, M. J. et al. Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat. Commun. 11, 3285 (2020).
Rogowski, A. et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Commun. 6, 7481 (2015).
Feng, J. et al. Polysaccharide utilization loci in Bacteroides determine population fitness and community-level interactions. Cell Host Microbe https://doi.org/10.1016/j.chom.2021.12.006 (2022).
Pollak, S. et al. Public good exploitation in natural bacterioplankton communities. Sci. Adv. 7, eabi4717 (2022).
Cuskin, F. et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517, 165–169 (2015).
Patnode, M. L. et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell 179, 59–73.e13 (2019).
Walter, J., Maldonado-Gómez, M. X. & Martínez, I. To engraft or not to engraft: an ecological framework for gut microbiome modulation with live microbes. Curr. Opin. Biotechnol. 49, 129–139 (2018).
Jernberg, C., Löfmark, S., Edlund, C. & Jansson, J. K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 1, 56–66 (2007).
Dethlefsen, L., Huse, S., Sogin, M. L. & Relman, D. A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6, e280 (2008).
Becattini, S., Taur, Y. & Pamer, E. G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478 (2016).
Shade, A. et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 3, 417 (2012).
Coyte, K. Z., Schluter, J. & Foster, K. R. The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666 (2015).
Stone, L. The stability of mutualism. Nat. Commun. 11, 2648 (2020).
Ratzke, C., Barrere, J. & Gore, J. Strength of species interactions determines biodiversity and stability in microbial communities. Nat. Ecol. Evol. 4, 376–383 (2020).
Butler, S. & O’Dwyer, J. P. Stability criteria for complex microbial communities. Nat. Commun. 9, 2970 (2018).
Li, W. & Stevens, M. H. H. Fluctuating resource availability increases invasibility in microbial microcosms. Oikos 121, 435–441 (2012).
Nobuhiko, K. et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329 (2012).
Maltby, R., Leatham-Jensen, M. P., Gibson, T., Cohen, P. S. & Conway, T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS ONE 8, e53957 (2013).
Leatham, M. P. et al. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect. Immun. 77, 2876–2886 (2009).
Venturelli, O. S. et al. Deciphering microbial interactions in synthetic human gut microbiome communities. Mol. Syst. Biol. 14, e8157 (2018).
Clark, R. L. et al. Design of synthetic human gut microbiome assembly and butyrate production. Nat. Commun. 12, 3254 (2021).
Hromada, S. et al. Negative interactions determine Clostridioides difficile growth in synthetic human gut communities. Mol. Syst. Biol. 17, e10355 (2021).
MacArthur, R. Species packing and competitive equilibrium for many species. Theor. Popul. Biol. 1, 1–11 (1970).
Ndeh, D. et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70 (2017).
Grondin, J. M., Tamura, K., Déjean, G., Abbott, D. W. & Brumer, H. Polysaccharide utilization loci: fueling microbial communities. J. Bacteriol. 199, e00860-16 (2017).
Haiser, H. J. et al. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 341, 295–298 (2013).
Devendran, S. et al. Clostridium scindens ATCC 35704: integration of nutritional requirements, the complete genome sequence, and global transcriptional responses to bile acids. Appl. Environ. Microbiol. 85, e00052-19 (2019).
Rey, F. E. et al. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl Acad. Sci. USA 110, 13582–13587 (2013).
Kaoutari, A. E., Armougom, F., Gordon, J. I., Raoult, D. & Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 11, 497–504 (2013).
Pereira, F. C. & Berry, D. Microbial nutrient niches in the gut. Environ. Microbiol. 19, 1366–1378 (2017).
Despres, J. et al. Xylan degradation by the human gut Bacteroides xylanisolvens XB1A(T) involves two distinct gene clusters that are linked at the transcriptional level. BMC Genomics 17, 326 (2016).
Déjean, G. et al. Synergy between cell surface glycosidases and glycan-binding proteins dictates the utilization of specific beta(1,3)-glucans by human gut bacteroides. mBio 11, e00095-20 (2020).
Hamaker, B. R. & Tuncil, Y. E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 426, 3838–3850 (2014).
Bishop, C. M. Pattern Recognition and Machine Learning (Information Science and Statistics) (Springer, 2006).
Wasserman, L. All of Statistics: A Concise Course in Statistical Inference (Springer Texts in Statistics) (Springer, 2003).
Willing, B. P., Russell, S. L. & Finlay, B. B. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
Panda, S. et al. Short-term effect of antibiotics on human gut microbiota. PLoS ONE 9, e95476 (2014).
Ng, K. M. et al. Recovery of the gut microbiota after antibiotics depends on host diet, community context, and environmental reservoirs. Cell Host Microbe 26, 650–665.e4 (2019).
Van der Waaij, D., Berghuis-de Vries, J. M. & Lekkerkerk-van der Wees, J. E. C. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hygiene 69, 405–411 (1971).
Freter, R. In vivo and in vitro antagonism of intestinal bacteria against Shigella flexneri. II. The inhibitory mechanism. J. Infect. Dis. 110, 38–46 (1962).
Maldonado-Gómez, M. X. et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 20, 515–526 (2016).
Sorbara, M. T. & Pamer, E. G. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 12, 1–9 (2019).
Litvak, Y. & Bäumler, A. J. The founder hypothesis: a basis for microbiota resistance, diversity in taxa carriage, and colonization resistance against pathogens. PLoS Pathog. 15, e1007563 (2019).
Jenior, M. L., Leslie, J. L., Young, V. B. & Schloss, P. D. Clostridium difficile colonizes alternative nutrient niches during infection across distinct murine gut microbiomes. mSystems 2, e00063-17 (2017).
Momose, Y., Hirayama, K. & Itoh, K. Competition for proline between indigenous Escherichia coli and E. coli O157:H7 in gnotobiotic mice associated with infant intestinal microbiota and its contribution to the colonization resistance against E. coli O157:H7. Antonie van Leeuwenhoek 94, 165–171 (2008).
Fabich, A. J. et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76, 1143–1152 (2008).
Shepherd, E. S., DeLoache, W. C., Pruss, K. M., Whitaker, W. R. & Sonnenburg, J. L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 557, 434–438 (2018).
Jenior, M. L., Leslie, J. L., Young, V. B. & Schloss, P. D. Clostridium difficilealters the structure and metabolism of distinct cecal microbiomes during initial infection to promote sustained colonization. mSphere 3, e00261-18 (2018).
Li, S., Tan, J., Yang, X., Ma, C. & Jiang, L. Niche and fitness differences determine invasion success and impact in laboratory bacterial communities. ISME J. 13, 402–412 (2019).
Deng, Y.-J. & Wang, S. Y. Synergistic growth in bacteria depends on substrate complexity. J. Microbiol. 54, 23–30 (2016).
Deng, Y.-J. & Wang, S. Y. Complex carbohydrates reduce the frequency of antagonistic interactions among bacteria degrading cellulose and xylan. FEMS Microbiol. Lett. 364, fnx019 (2017).
Wu, F. et al. Modulation of microbial community dynamics by spatial partitioning. Nat. Chem. Biol. 18, 394–402 (2022).
Åström, K. J. & Murray, R. Feedback Systems. An Introduction for Scientists and Engineers (Princeton Univ. Press, 2008).
Hammarlund, S. P. & Harcombe, W. R. Refining the stress gradient hypothesis in a microbial community. Proc. Natl Acad. Sci. USA 116, 15760–15762 (2019).
Pacheco, A. R., Osborne, M. L. & Segrè, D. Non-additive microbial community responses to environmental complexity. Nat. Commun. 12, 2365 (2021).
Dal Bello, M., Lee, H., Goyal, A. & Gore, J. Resource–diversity relationships in bacterial communities reflect the network structure of microbial metabolism. Nat. Ecol. Evol. 5, 1424–1434 (2021).
Magnúsdóttir, S. et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 35, 81–89 (2017).
Baranwal, M. et al. Recurrent neural networks enable design of multifunctional synthetic human gut microbiome dynamics. eLife 11, e73870 (2022).
Palleja, A. et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3, 1255–1265 (2018).
Dethlefsen, L. & Relman, D. A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108, 4554–4561 (2011).
Ramirez, J. et al. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 10, 572912 (2020).
Zhang, J., Kobert, K., Flouri, T. & Stamatakis, A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620 (2014).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Raue, A. et al. Lessons learned from quantitative dynamical modeling in systems biology. PLoS ONE 8, e74335 (2013).
Babtie, A. C., Kirk, P. & Stumpf, M. P. H. Topological sensitivity analysis for systems biology. Proc. Natl Acad. Sci. USA 111, 18507–18512 (2014).
Munsky, B., Hlavacek, W. S. & Tsimring, L. S. Quantitative Biology. Theory, Computational Methods, and Models (MIT Press, 2018).
Ashyraliyev, M., Fomekong-Nanfack, Y., Kaandorp, J. A. & Blom, J. G. Systems biology: parameter estimation for biochemical models. FEBS J. 276, 886–902 (2009).
Ravcheev, D. A., Godzik, A., Osterman, A. L. & Rodionov, D. A. Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: comparative genomics reconstruction of metabolic and regulatory networks. BMC Genomics 14, 873 (2013).
Salyers, A. A., Vercelloitti, J. R., West, S. E. & Wilkins, T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 33, 319–322 (1977).
Sun, X., Liu, Y., Jiang, P., Song, S. & Ai, C. Interaction of sulfated polysaccharides with intestinal Bacteroidales plays an important role in its biological activities. Int. J. Biol. Macromol. 168, 496–506 (2021).
Respondek, F. et al. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE 8, e71026 (2013).
Schwiertz, A. et al. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 25, 46–51 (2002).
Benítez-Páez, A., Moreno, F. J., Sanz, M. L. & Sanz, Y. Genome structure of the symbiont Bifidobacterium pseudocatenulatum CECT 7765 and gene expression profiling in response to lactulose-derived oligosaccharides. Front. Microbiol. 7, 624 (2016).
Bernalier, A., Willems, A., Leclerc, M., Rochet, V. & Collins, M. D. Ruminococcus hydrogenotrophicus sp. nov., a new H2/CO2-utilizing acetogenic bacterium isolated from human feces. Arch. Microbiol. 166, 176–183 (1996).
Moshfegh, A. J., Friday, J. E., Goldman, J. P. & Ahuja, J. K. C. Presence of inulin and oligofructose in the diets of Americans. J. Nutr. 129, 1407S–1411S (1999).
Sonnenburg, E. D. et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell 141, 1241–1252 (2010).
Devillé, C., Damas, J., Forget, P., Dandrifosse, G. & Peulen, O. Laminarin in the dietary fibre concept. J. Sci. Food Agric. 84, 1030–1038 (2004).
Selvendran, R. R. The plant cell wall as a source of dietary fiber: chemistry and structure. Am. J. Clin. Nutr. 39, 320–337 (1984).
Acknowledgements
We thank R. Clark and B. Connors for helpful suggestions and comments on this work and manuscript, and S. Hromada for helpful suggestions and assistance with data analysis. This research was supported by the National Institutes of Health under grant number R35GM124774. E.O.L. was supported by an NHGRI training grant to the Genomic Sciences Training Program 5T32HG002760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
O.S.V. and E.O.L. designed the study. E.O.L performed the experiments. E.O.L. and O.S.V. analysed the data. J.T., P.L.K.C. and Y.Q. developed code for model fitting and performed computational analyses. E.O.L and P.L.K.C. performed resource model simulations. E.O.L. and O.S.V. wrote the manuscript and all authors contributed to its revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work.Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Growth responses of individual species in the presence of different carbohydrates determines carbohydrate responder or non-responder metabolic niche classification.
(a) Schematic representing species’ growth response as a function of carbohydrate concentration. A carbohydrate responder is defined as a species whose total growth (area under the curve or intOD) is significantly higher in the presence of at least two concentrations of carbohydrate than in the absence of carbohydrate (two-sided unpaired t-test, p-value<0.05) as described in the Methods. (b) Relationship between carbohydrate concentration and total monospecies growth. Species were grown in the presence of four concentrations (x-axis) of each carbohydrate indicated by the color of each datapoint or in the absence of carbohydrate. Square and circle data points denote the average intOD for each species or individual biological replicates, respectively. All conditions had n = 3 biological replicates. Concentrations where the intOD was significantly greater in carbohydrate containing media than in the absence of carbohydrate are denoted by asterisk (significance was determined as described in (A)). Exact p-values for each condition are in Supplementary Table 2). Lower right: boxplot of the number of concentrations with a significantly higher intOD relative to the no carbohydrate control. The color of the datapoint represents the specific carbohydrate. In each boxplot, the horizontal lines represent the first quartile, median, and third quartile from bottom to top and upper and lower whiskers represent the minimum and maximum, respectively. The threshold for classification as a carbohydrate responder is denoted by the dashed line. (c) Left: Scatter plot of carbohydrate complexity versus the number of carbohydrate responders (Methods). Right: scatter plot of carbohydrate complexity versus the sum of individual species growth (intOD). Datapoints denote different carbohydrate types. Spearman rho and p-values are indicated in the upper right corner of each scatter plot. The dashed line in both plots indicates the linear regression line. (d) Scatter plot of carbohydrate concentration versus the sum of individual species growth (intOD). Each datapoint represents the sum of each average intOD of all biological replicates (n = 3 for each species). The dashed line indicates the linear regression line.
Extended Data Fig. 2 Carbohydrate complexity and reduced carbohydrate concentrations drive community assembly towards a no carbohydrate state.
(a) Stacked bar plot of species absolute abundance in the presence of individual carbohydrates at each passage. Individual bars denote biological replicates and each group of bars for a given carbohydrate denotes a distinct passage. (b) Scatter plots of species absolute abundance in the presence of a given fibre versus the constituent sugars at the final 24-hour growth cycle (that is, passage 4). Each biological replicate is shown as a smaller datapoint connected by a colored line to the mean (large data point). Datapoints and lines are colored according to species. Dashed line denotes the linear regression line. (c) Stacked bar plot of species absolute abundance in the presence of varied concentrations of individual carbohydrates at the final 24-hour passage. Individual bars denote biological replicates and each group of bars denotes a different carbohydrate concentration. Concentrations are 10, 5, 2.5, and 1.25 g/L from left to right. (d) Principal component analysis of the relative species abundances in Comm10 grown in varied concentrations of each carbohydrate. Colored arrows indicate the shift in community composition with decreasing carbohydrate concentration. The dashed circle represents clusters identified by k-means. The PCA loadings (coefficients of the linear combination of the original species variables from which the principal components are constructed) are represented by the black lines. (e) Relationship between carbohydrate concentration and Euclidean distance of species relative abundances in the presence of each fibre (10 g/L) and corresponding sugars as a function of sugar concentration. Small circular data points represent the Euclidean distance between each pair of biological replicates, and the large square represents the mean. A two-sided unpaired t-test was used to determine the statistical significance between communities grown in each concentration of sugar and the highest concentration of fibre. All p-values < 0.05 are specified above the plot.
Extended Data Fig. 3 Assembly of a community excluding the non-responders DP and EL yields similar trends to Comm10 in response to variation in carbohydrate concentration.
(a) Stacked bar plots of species absolute abundance in an 8-member community excluding DP and EL (all species in Comm10 except DP and EL) in the presence of a different concentrations of the constituent sugars of each fibre or corresponding fibre at the final passage (that is, passage 4). Individual bars denote biological replicates and different groups for a given carbohydrate represent different concentrations (10, 5, 2.5, and 1.25 g/L). (b) Relationship between carbohydrate concentration and the Euclidean distance in community composition of the 8-member community in the presence of the constituent sugars or corresponding fibre (10 g/L) as a function of sugar concentration at the final passage. Small circular data points represent the Euclidean distance between each pair of biological replicates from different conditions and the large square represents the mean. A two-sided unpaired t-test evaluated the statistical significance between the Euclidean distances of communities grown in each concentration of sugar and the highest concentration of fibre. All p-values < 0.05 are specified above the plot.
Extended Data Fig. 4 Inter-species interactions are major determinants of community assembly across carbohydrate types.
(a) Scatter plots of predicted species relative abundances using fitted null models (logistic growth model fit to single species growth responses) and experimental measurements at 24 hours (that is, passage 1). Each small circle represents a single biological replicate and the large transparent circles represents the mean. The dashed line indicates the linear regression line. When removing the outlier AC in laminarin sugars, the correlation vanishes (Spearman 𝜌= −0.068 and p = 7.36e-1), indicating that AC drives the correlation. When removing the data point for BT in xylan sugars, the strength of the correlation substantially decreases (Spearman 𝜌= 0.46 and p = 1.55e-2), demonstrating that the BT is a major driver of the correlation. (b) Categorial scatter plots of species absolute abundance in a set of subcommunities containing different combinations of responder and non-responder species measured at 24 hours. Each datapoint represents a single biological replicate (the number of biological replicates for each condition is listed in Supplementary Table 3) and the color indicates the initial richness of the designed subcommunity (number of species introduced at the beginning of the experiment). Responder two-letter codes are indicated by underlined text. In each boxplot, the horizontal lines represent the first quartile, median, and third quartile from bottom to top and upper and lower whiskers denote the minimum and maximum, respectively.
Extended Data Fig. 5 Complex carbohydrates promote a balance between positive and negative inter-species interactions.
(a) Histogram represents the Posterior parameter distributions for each interaction coefficient aij in each gLV model (mean is given by equation 6 and covariance is given by equation 13 in the Supplementary Information) The color of the distribution indicates the magnitude of the median (indicated at the bottom of each subplot), with red denoting a negative value and blue denoting a positive value. Effector species are indicated on the x-axis and recipient species are indicated on the y-axis. (b) Scatter plots of measured versus predicted for each gLV model. Each data point represents measured absolute abundance (x-axis) and the corresponding model prediction after running 10-fold cross validation (y-axis). Error bars in the x-direction represent 1 s.d. from the mean of biological replicates (the number of biological replicates for each condition in listed in Supplementary Table 3). Error bars in the y-direction represent 1 s.d. of the model prediction given by equation 18 in the Supplementary Text. Briefly the model’s estimated variance reflects uncertainty due to measurement noise and the variation in the parameter estimates. Datapoints are colored by carbohydrate. In each subplot, the solid line indicates x = y and the dashed line indicates the linear regression line. (c) Stacked bar plot of the proportion of positive and negative inferred inter-species interactions (90 total). (d) Stacked bar plot of the number of positive and negative inferred inter-species interaction coefficients with a p-value<0.05 according to the Wald test. Exact p-values can be found in Supplementary Table 4. (e) Bar plot of the ratio in the number of positive and negative inferred interactions in the presence (left bar within each group) and absence (right bar within each grop) of the Wald test. The dashed line indicates an equal ratio of positive and negative interactions.
Extended Data Fig. 6 Growth limitation and carbohydrate complexity influence the reproducibility of community assembly in response to the variation in initial species abundances.
(a) Stacked bar plot of species relative abundance in Comm10 inoculated with different initial species abundances in the presence a single carbohydrate measured at the final passage. Each bar represents a single biological replicate and the label beneath each group indicates the single species in the inoculum with the highest initial abundance. (b) Scatter plot of carbohydrate complexity versus the pairwise Euclidean distance in community composition of conditions inoculated with different initial species abundances. Each small circular datapoint represents the Euclidean distance between each pair of biological replicates for each passage (four 24-hour growth cycles), and the large square data point represents the mean across all replicate pairs. The number of biological replicates in each condition are visualized in (A). The dashed line indicates the linear regression line. (c) Scatter plot of the average monospecies growth (intOD) summed across all Comm10 species and pairwise Euclidean distance between communities inoculated with different initial species abundances. Each small circular datapoint represents the Euclidean distance between each pair of biological replicates for each passage, and the large square data point represents the mean across all replicate pairs. The number of biological replicates in each condition is visualized in (A). The dashed line indicates the linear regression line.
Extended Data Fig. 7 The gLV and MacArthur’s consumer-resource model demonstrate negative inter-species interactions drive the sensitivity of community assembly to variation in initial species abundances.
(a) Categorial scatter plot of the pairwise Euclidean distance between communities based on gLV simulations using the inferred parameter sets. For each parameter set, the gLV model was simulated from different initial conditions mirroring the passaging experimental design. Each data point represents the pairwise Euclidean distance of the community composition at the endpoint of each passage. A two-sided unpaired t-test evaluated the statistical significance between the distribution of pairwise Euclidean distances in each fibre and the constitutive sugars across all 24-hr passages. P-values <0.05 are displayed above the plot.. For all boxplots in this figure, the horizontal lines on the boxplot represents the first quartile, median, and third quartile from bottom to top and upper and lower whiskers representing the minimum and maximum, respectively. (b) Categorial scatter plot of the Euclidean distance between communities based on gLV simulations using randomly sampled parameters (Methods). A two-sided unpaired t-test evaluated the statistical significance between distances in each fibre from the respective sugars for the endpoints of all passages. P-values <0.05 are displayed above the plot. (c) The median of the normal distribution used for generating random parameter sampling and the proportion of negative and positive inter-species interaction coefficients tallied for each parameter set. (d) Categorical scatter plot of the Euclidean distance between communities based on gLV simulations using randomly sampled parameters with only negative interaction coefficients (Methods). A two-sided unpaired t-test evaluated the statistical significance between distances in fibre and the constitutive sugars for each 24-hour passage. P-values < 0.05 are displayed above the plot. (e) Relationship between the median of the normal distribution used for generating random parameter sampling and the proportion of inter-species interaction coefficients that were negative or zero tallied for each parameter set. (f) The distribution of Euclidean distances between pairs of communities with different initial species abundances using the MacArthur’s consumer resource model simulations (Methods). Each set of 10 boxplots represents models with a unique number of carbohydrate responders (Methods). The color of each box denotes the median magnitude of carbohydrate utilization parameters randomly chosen from a normal distribution.
Extended Data Fig. 8 The growth of 16 additional diverse human gut species is limited by carbohydrate complexity consistent with the trends for Comm10.
(a) Phylogenetic tree of 26 species used to invade resident communities, including the 10 species in Comm10. Comm10 species are indicated by colored bacteria shapes. The phylogenetic tree was generated based on concatenated alignment of 37 marker genes using Phylosift. (b) Heatmap of the average integral OD600 (intOD) of individual invader species grown in media containing each carbohydrate type (n = 3 biological replicates). For each carbohydrate, monospecies growth was characterized in the presence of 5 or 10 g/L (top wedges indicate concentrations). Carbohydrates are ordered by carbohydrate complexity. The total growth of each species (area under the curve or intOD) for each carbohydrate was hierarchically clustered based on Euclidean distance. (c) Scatter plot of carbohydrate complexity versus the sum of monospecies growth (sum of intOD) for all species in the expanded 26-member community. Datapoints denote the summed average intOD of each species across biological replicates (n = 3). The dashed line indicates the linear regression line.
Extended Data Fig. 9 The resistance of Comm10 to invasion was enhanced in the presence of complex carbohydrates or low carbohydrate concentrations.
(a) Stacked bar plots of species absolute abundances in Comm10 measured at the final passage. Individual bars denote biological replicates and different groups denote varying concentrations for a given carbohydrate. Hashed bars denote invader species. (b) Categorial scatter plot of the difference in species absolute abundance the presence and absence of invasion. Specifically, the difference in species abundance was computed by subtracting the mean abundance of each species in the invaded communities from the mean abundance in communities that were not invaded at the final passage. The number of biological replicates in each condition is shown in (A). (c) Scatter plot of monospecies growth (intOD) summed across all 10 species in the highest concentration (10 g/L) of each carbohydrate type versus the Euclidean distance of the composition of Comm10 in the presence and absence of invasion. The circular datapoints represent biological replicates and the square represents the mean of all replicates. The dashed line indicates the linear regression line. (d) Scatter plot of monospecies growth (intOD) summed across all 10 species versus Euclidean distance of the composition of Comm10 in the presence and absence of invasion for different carbohydrate concentrations (10, 5, 2.5 and 1.25 g/L) indicated by the size of the datapoint. The circular datapoints represent biological replicates and the square represents the mean of all replicates. The dashed line indicates the linear regression line. (e) Stacked bar plots of the absolute abundance of species in Comm10 in the presence of different carbohydrate concentrations measured at the final passage. The un-invaded control communities and invaded communities are on the left and right of each group of stacked bar plots, respectively. Individual bars denote biological replicates. Hashed bars denote invading species. (f) Categorial scatter plot of the Euclidean distance of species’ relative abundance in Comm10 in the presence and absence of invasion for different carbohydrate concentrations. Each datapoint represents the distance between each biological replicate A two-sided unpaired t-test evaluated the statistical significance between the distribution of pairwise Euclidean distances between different carbohydrate concentrations. P-values < 0.05 are displayed above the line connecting each pair of concentrations.
Extended Data Fig. 10 The degree of resource competition for a given carbohydrate impacts the resistance of the resident community to invasion.
(a) Categorial scatter plot of the average total growth (intOD) of monospecies (n = 3) over a 24 h period. The shape of each datapoint indicates inclusion of this species in the designed communities and each box represents each designed community. (b) Categorial scatter plot of the Euclidean distance of the resident community in the presence and absence of invasion for each biological replicate. A two-sided unpaired t-test evaluated the statistical significance between each designed community. P-values <0.05 are shown above the line connecting each pair of conditions. The number of biological replicates is shown as stacked bars in (C). (c) Stacked bar plots of species absolute abundance in communities that were not invaded (left) or invaded (right) in the presence of each carbohydrate. Individual bars denote biological replicates and groups of bars denote different designed communities for a given carbohydrate. Invader species are denoted by hashed bars. (d) Categorial scatter plot of the total growth (intOD) of species used to invade Comm10 in each carbohydrate type. Species with the greatest absolute abundance in the community following invasion at the final passage are denoted by their respective 2-letter code associated to the given datapoint. The number of biological replicates are visualized as stacked bars in (C). (e) Categorial scatter plot of the mean absolute abundance (n = 3) of each species in the 26-member community inoculated with equal proportions after the final passage. The color and shape of the datapoint signifies the carbohydrate and type of designed resident community, respectively. (f) Relationship between summed growth of monospecies (sum of intOD) present in the resident community and the Euclidean distance in the presence and absence of invasion. Each individual species intOD over 24 h was averaged and then summed across all species in the given resident community Small datapoints represent the Euclidean distance between each pairwise replicate, and large data points represent the mean. (g) Scatter plot of invader abundance versus Euclidean distance of the resident community in the presence and absence of invasion. The color of datapoint indicates carbohydrate type, shape indicates the type of designed resident community, and size indicates carbohydrate concentration according to the legend. Datapoints represent the average of biological replicates and error bars represent 1 s.d. from the mean. The y-axis contains more measurements since the pairwise distance of each replicate was calculated (for example 3 biological replicates yield 9 Euclidean distances), hence averages and not individual biological replicates were shown. The dashed line indicates the linear regression line.
Supplementary information
Supplementary Information
Supplementary Methods 1–7.
Supplementary Tables 1–4
All supplementary tables, with each tab representing a separate table labelled as referenced in the manuscript text.
Source data
Source Data Fig. 1
Monospecies OD measurements and processed 16S rRNA sequencing data.
Source Data Fig. 2
Processed 16S rRNA sequencing data and gLV parameter distributions and statistics.
Source Data Fig. 3
Processed 16S rRNA sequencing data and model simulations.
Source Data Fig. 4
Processed 16S rRNA sequencing data.
Source Data Extended Data Fig. 1
Monospecies OD measurements.
Source Data Extended Data Fig. 2
Processed 16S rRNA sequencing data.
Source Data Extended Data Fig. 3
Processed 16S rRNA sequencing data.
Source Data Extended Data Fig. 4
Processed 16S rRNA sequencing data.
Source Data Extended Data Fig. 5
gLV parameter distributions and statistics.
Source Data Extended Data Fig. 6
Processed 16S rRNA sequencing data and model simulations.
Source Data Extended Data Fig. 7
gLV and MCR model simulations.
Source Data Extended Data Fig. 8
Monospecies OD measurements.
Source Data Extended Data Fig. 9
Processed 16S rRNA sequencing data.
Source Data Extended Data Fig. 10
Processed 16S rRNA sequencing data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ostrem Loss, E., Thompson, J., Cheung, P.L.K. et al. Carbohydrate complexity limits microbial growth and reduces the sensitivity of human gut communities to perturbations. Nat Ecol Evol 7, 127–142 (2023). https://doi.org/10.1038/s41559-022-01930-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01930-9
This article is cited by
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)