Abstract
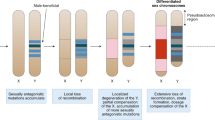
Contrary to classic theory prediction, sex-chromosome homomorphy is prevalent in the animal kingdom but it is unclear how ancient homomorphic sex chromosomes avoid chromosome-scale degeneration. Molluscs constitute the second largest, Precambrian-originated animal phylum and have ancient, uncharacterized homomorphic sex chromosomes. Here, we profile eight genomes of the bivalve mollusc family of Pectinidae in a phylogenetic context and show 350 million years sex-chromosome homomorphy, which is the oldest known sex-chromosome homomorphy in the animal kingdom, far exceeding the ages of well-known heteromorphic sex chromosomes such as 130–200 million years in mammals, birds and flies. The long-term undifferentiation of molluscan sex chromosomes is potentially sustained by the unexpected intertwined regulation of reversible sex-biased genes, together with the lack of sexual dimorphism and occasional sex chromosome turnover. The pleiotropic constraint of regulation of reversible sex-biased genes is widely present in ancient homomorphic sex chromosomes and might be resolved in heteromorphic sex chromosomes through gene duplication followed by subfunctionalization. The evolutionary dynamics of sex chromosomes suggest a mechanism for ‘inheritance’ turnover of sex-determining genes that is mediated by translocation of a sex-determining enhancer. On the basis of these findings, we propose an evolutionary model for the long-term preservation of homomorphic sex chromosomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All sequencing data have been deposited to the NCBI’s SRA database and GenBank under the project accession number PRJNA796071. The accession numbers are listed in Supplementary Table 2. The genome assemblies and functional annotations of scallop species are also available in the MolluscDB database (http://mgbase.qnlm.ac/page/download/download). Source data are provided with this paper.
Code availability
The software and codes used in this study are publicly available, with corresponding versions indicated in Methods.
References
Charlesworth, B. The evolution of sex chromosomes. Science 251, 1030–1033 (1991).
Wright, A. E., Dean, R., Zimmer, F. & Mank, J. E. How to make a sex chromosome. Nat. Commun. 7, 12087 (2016).
Jablonka, E. & Lamb, M. J. The evolution of heteromorphic sex chromosomes. Biol. Rev. Camb. Philos. Soc. 65, 249–276 (1990).
Charlesworth, D., Charlesworth, B. & Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118–128 (2005).
Abbott, J. K., Nordén, A. K. & Hansson, B. Sex chromosome evolution: historical insights and future perspectives. Proc. Biol. Sci. 284, 20162806 (2017).
Daish, T. & Grützner, F. Evolution and meiotic organization of heteromorphic sex chromosomes. Curr. Top. Dev. Biol. 134, 1–48 (2019).
Steinemann, S. & Steinemann, M. Y chromosomes: born to be destroyed. BioEssays 27, 1076–1083 (2005).
Eggert, C. Sex determination: the amphibian models. Reprod. Nutr. Dev. 44, 539–549 (2004).
Devlin, R. H. & Nagahama, Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208, 191–364 (2002).
Thiriot-Quievreux, C. Advances in chromosomal studies of gastropod molluscs. J. Molluscan Stud. 69, 187–202 (2003).
Breton, S., Capt, C., Guerra, D. & Stewart, D. in Transitions Between Sexual Systems (ed. Leonard, J. L.) 165–192 (Springer International, 2007).
Otto, S. P. et al. About PAR: the distinct evolutionary dynamics of the pseudoautosomal region. Trends Genet. 27, 358–367 (2011).
Yazdi, H. P. & Ellegren, H. Old but not (so) degenerated—slow evolution of largely homomorphic sex chromosomes in ratites. Mol. Biol. Evol. 31, 1444–1453 (2014).
Kuhl, H. et al. A 180 Myr-old female-specific genome region in sturgeon reveals the oldest known vertebrate sex determining system with undifferentiated sex chromosomes. Philos. Trans. R. Soc. Lond. B 376, 20200089 (2021).
Furman, B. L. S. et al. Sex chromosome evolution: so many exceptions to the rules. Genome Biol. Evol. 12, 750–763 (2020).
Bachtrog, D. et al. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 (2014).
Charlesworth, D. When and how do sex–linked regions become sex chromosomes? Evolution 75, 569–581 (2021).
Vicoso, B. Molecular and evolutionary dynamics of animal sex–chromosome turnover. Nat. Ecol. Evol. 3, 1632–1641 (2019).
Palmer, D. H., Rogers, T. F., Dean, R. & Wright, A. E. How to identify sex chromosomes and their turnover. Mol. Ecol. 28, 4709–4724 (2019).
Giese, A. C. & Pearse, J. S. (eds) Reproduction of Marine Invertebrates Vol. 4 (Academic Press, 1977).
Zhang, N., Xu, F. & Guo, X. Genomic analysis of the Pacific oyster (Crassostrea gigas) reveals possible conservation of vertebrate sex determination in a mollusc. G3 4, 2207–2217 (2014).
Evensen, K. G., Robinson, W. E., Krick, K., Murray, H. M. & Poynton, H. C. Comparative phylotranscriptomics reveals putative sex differentiating genes across eight diverse bivalve species. Comp. Biochem Physiol. D 41, 100952 (2022).
Budd, G. E. The earliest fossil record of the animals and its significance. Philos. Trans. R. Soc. Lond. B 363, 1425–1434 (2008).
Collin, R. Phylogenetic patterns and phenotypic plasticity of molluscan sexual systems. Integr. Comp. Biol. 53, 723–735 (2013).
Wallace, C. Parthenogenesis, sex, and chromosomes in Potamopyrgus. J. Molluscan Stud. 58, 93–107 (1992).
Guo, X. & Allen, S. K. Jr. Sex determination and polyploid gigantism in the dwarf surfclam (Mulinia lateralis Say). Genetics 138, 1199–1206 (1994).
Jiao, W. et al. High-resolution linkage and quantitative trait locus mapping aided by genome survey sequencing: building up an integrative genomic framework for a bivalve mollusc. DNA Res. 21, 85–101 (2014).
Plazzi, F. & Passamonti, M. Towards a molecular phylogeny of mollusks: bivalves’ early evolution as revealed by mitochondrial genes. Mol. Phylogenet. Evol. 57, 641–657 (2010).
Serb, J. M. Reconciling morphological and molecular approaches in developing a phylogeny for the Pectinidae (Mollusca: Bivalvia). Dev. Aquac. Fish. Sci. 40, 1–29 (2016).
Baird, G. C. & Brett, C. Regional variation and paleontology of two coral beds in the Middle Devonian Hamilton Group of western New York. J. Paleontol. 57, 417–446 (1983).
Mergl, M., Massa, D. & Plauchut, B. Devonian and Carboniferous brachiopods and bivalves of the Djado sub-basin (north Niger, SW Libya). J. Czech. Geol. Soc. 46, 169–188 (2001).
Sun, W. & Gao, L. Phylogeny and comparative genomic analysis of Pteriomorphia (Mollusca Bivalvia) based on complete mitochondrial genomes. Mar. Biol. Res. 13, 255–268 (2017).
Waller, T. R. New Phylogenies of the Pectinidae (Mollusca: Bivalvia): reconciling morphological and molecular approaches. Dev. Aquac. Fish. Sci. 35, 1–44 (2006).
Alejandrino, A., Puslednik, L. & Serb, J. M. Convergent and parallel evolution in life habit of the scallops (Bivalvia: Pectinidae). BMC Evol. Biol. 11, 164 (2011).
Leder, E. H. et al. Female-biased expression on the X chromosome as a key step in sex chromosome evolution in threespine sticklebacks. Mol. Biol. Evol. 27, 1495–1503 (2010).
Meisel, R. P., Malone, J. H. & Clark, A. G. Disentangling the relationship between sex-biased gene expression and X-linkage. Genome Res. 22, 1255–1265 (2012).
Albritton, S. E. et al. Sex-biased gene expression and evolution of the x chromosome in nematodes. Genetics 197, 865–883 (2014).
Li, L. et al. Construction of AFLP-based genetic linkage map for Zhikong scallop, Chlamys farreri Jones et Preston and mapping of sex-linked markers. Aquaculture 245, 63–73 (2005).
Boulanger, L. et al. Foxl2 is a female sex-determining gene in the goat. Curr. Biol. 24, 404–408 (2014).
Zhu, L. et al. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev. 14, 1750–1764 (2015).
Goldstone, J. V. et al. Genetic and structural analyses of cytochrome P450 hydroxylases in sex hormone biosynthesis: sequential origin and subsequent coevolution. Mol. Phylogenet. Evol. 94, 676–687 (2016).
Bertho, S. et al. Foxl2 and its relatives are evolutionary conserved players in gonadal sex differentiation. Sex. Dev. 10, 111–129 (2016).
Capt, C. et al. Deciphering the link between doubly uniparental inheritance of mtDNA and sex determination in bivalves: clues from comparative transcriptomics. Genome Biol. Evol. 10, 577–590 (2018).
Kirkpatrick, M. How and why chromosome inversions evolve. PLoS Biol. 8, e1000501 (2010).
Natri, H. M., Merilä, J. & Shikano, T. The evolution of sex determination associated with a chromosomal inversion. Nat. Commun. 10, 145 (2019).
Rubtsov, N. B. et al. Reorganization of the X chromosome in voles of the genus Microtus. Cytogenet. Genome Res. 99, 323–329 (2002).
Kalvari, I. et al. Non-Coding RNA analysis using the Rfam database. Curr. Protoc. Bioinforma. 62, e51 (2018).
The RNAcentral Consortium. RNAcentral: a hub of information for non-coding RNA sequences. Nucleic Acids Res. 47, D221–D229 (2019).
Esnault, C., Maestre, J. & Heidmann, T. Human LINE retrotransposons generate processed pseudogenes. Nat. Genet. 24, 363–367 (2000).
Troskie, R. L., Faulkner, G. J. & Cheetham, S. W. Processed pseudogenes: a substrate for evolutionary innovation: retrotransposition contributes to genome evolution by propagating pseudogene sequences with rich regulatory potential throughout the genome. BioEssays 43, e2100186 (2021).
Kostmann, A., Kratochvíl, L. & Rovatsos, M. Poorly differentiated XX/XY sex chromosomes are widely shared across skink radiation. Proc. Biol. Sci. 288, 20202139 (2021).
Veyrunes, F. et al. Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res. 18, 965–973 (2008).
Vicoso, B. & Bachtrog, D. Numerous transitions of sex chromosomes in Diptera. PLoS Biol. 13, e1002078 (2015).
Panigrahi, A. & O’Malley, B. W. Mechanisms of enhancer action: the known and the unknown. Genome Biol. 22, 108 (2021).
Braendle, C. & Félix, M. A. Sex determination: ways to evolve a hermaphrodite. Curr. Biol. 16, R468–R471 (2006).
Ellegren, H. & Parsch, J. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 8, 689–698 (2007).
Wang, S. et al. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat. Ecol. Evol. 1, 120 (2017).
Silina, A. V. Is sexual size dimorphism inherent in the scallop Patinopecten yessoensis? Scientifica 2016, 9 (2016).
Yoshimura, T. et al. Sexual dimorphism in shell growth of the oviparous boreal scallop Swiftopecten swiftii (Bivalvia: Pectinidae). J. Molluscan Stud. 85, 253–261 (2019).
Mank, J. E., Nam, K., Brunström, B. & Ellegren, H. Ontogenetic complexity of sexual dimorphism and sex-specific selection. Mol. Biol. Evol. 27, 1570–1578 (2010).
Perry, J. C., Harrison, P. W. & Mank, J. E. The ontogeny and evolution of sex-biased gene expression in Drosophila melanogaster. Mol. Biol. Evol. 31, 1206–1219 (2014).
Xu, W. et al. Transcriptomic analysis revealed gene expression profiles during the sex differentiation of Chinese tongue sole (Cynoglossus semilaevis). Comp. Biochem Physiol. D 40, 100919 (2021).
Ayers, K. L. et al. Identification of candidate gonadal sex differentiation genes in the chicken embryo using RNA-seq. BMC Genomics 16, 704 (2015).
Aivatiadou, E., Mattei, E., Ceriani, M., Tilia, L. & Berruti, G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Mol. Biol. Cell. 18, 1530–1542 (2007).
Li, J., Xia, F. & Li, W. X. Coactivation of STAT and Ras is required for germ cell proliferation and invasive migration in Drosophila. Dev. Cell 5, 787–798 (2003).
Chowdhury, I., Branch, A., Mehrabi, S., Ford, B. D. & Thompson, W. E. Gonadotropin-dependent neuregulin-1 signaling regulates female rat ovarian granulosa cell survival. Endocrinology 158, 3647–3660 (2017).
Windley, S. P. & Wilhelm, D. Signaling pathways involved in mammalian sex determination and gonad development. Sex. Dev. 9, 297–315 (2015).
Zhou, Q. et al. Complex evolutionary trajectories of sex chromosomes across bird taxa. Science 346, 1246338 (2014).
Vicoso, B., Kaiser, V. B. & Bachtrog, D. Sex-biased gene expression at homomorphic sex chromosomes in emus and its implication for sex chromosome evolution. Proc. Natl Acad. Sci. USA 110, 6453–6458 (2013).
Itoh, Y. et al. Dosage compensation is less effective in birds than in mammals. J. Biol. 6, 2 (2007).
Mank, J. E. & Ellegren, H. All dosage compensation is local: gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity 102, 312–320 (2009).
McQueen, H. A. & Clinton, M. Avian sex chromosomes: dosage compensation matters. Chromosome Res. 17, 687–697 (2009).
Emerson, J. J., Kaessmann, H., Betrán, E. & Long, M. Extensive gene traffic on the mammalian X chromosome. Science 303, 537–540 (2004).
Vibranovski, M. D., Zhang, Y. & Long, M. General gene movement off the X chromosome in the Drosophila genus. Genome Res. 19, 897–903 (2009).
Baker, R. H., Narechania, A., Johns, P. M. & Wilkinson, G. S. Gene duplication, tissue-specific gene expression and sexual conflict in stalk-eyed flies (Diopsidae). Philos. Trans. R. Soc. Lond. B 367, 2357–2375 (2012).
Edgecombe, J., Urban, L., Todd, E. V. & Gemmell, N. J. Might gene duplication and neofunctionalization contribute to the sexual lability observed in fish? Sex. Dev. 15, 122–133 (2021).
Mank, J. E., Hultin-Rosenberg, L., Zwahlen, M. & Ellegren, H. Pleiotropic constraint hampers the resolution of sexual antagonism in vertebrate gene expression. Am. Nat. 171, 35–43 (2008).
Gallach, M. & Betrán, E. Intralocus sexual conflict resolved through gene duplication. Trends Ecol. Evol. 26, 222–228 (2011).
Perry, J. C. Duplication resolves conflict. Nat. Ecol. Evol. 2, 597–598 (2018).
Yoshimoto, S. et al. Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system. Development 137, 2519–2526 (2010).
Wang, Z. et al. Phylogeny and sex chromosome evolution of Palaeognathae. J. Genet. Genom. 49, 109–119 (2021).
Wyman, M. J., Cutter, A. D. & Rowe, L. Gene duplication in the evolution of sexual dimorphism. Evolution 66, 1556–1566 (2012).
Rodriguez-Caro, F. et al. Novel doublesex duplication associated with sexually dimorphic development of dogface butterfly wings. Mol. Biol. Evol. 38, 5021–5033 (2021).
Bergero, R. & Charlesworth, D. The evolution of restricted recombination in sex chromosomes. Trends Ecol. Evol. 24, 94–102 (2009).
Gamble, T. et al. Restriction site–associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Mol. Biol. Evol. 32, 1296–1309 (2015).
Jeffries, D. L. et al. A rapid rate of sex-chromosome turnover and non-random transitions in true frogs. Nat. Commun. 9, 4088 (2018).
Woram, R. A. et al. Comparative genome analysis of the primary sex-determining locus in salmonid fishes. Genome Res. 13, 272–280 (2003).
Perrin, N. Sex reversal: a fountain of youth for sex chromosomes? Evolution 63, 3043–3049 (2009).
Xu, L., Wa Sin, S. Y., Grayson, P., Edwards, S. V. & Sackton, T. B. Evolutionary dynamics of sex chromosomes of paleognathous birds. Genome Biol. Evol. 11, 2376–2390 (2019).
VanKuren, N. W. & Long, M. Gene duplicates resolving sexual conflict rapidly evolved essential gametogenesis functions. Nat. Ecol. Evol. 2, 705–712 (2018).
Lipinska, A. et al. Sexual dimorphism and the evolution of sex-biased gene expression in the brown alga Ectocarpus. Mol. Biol. Evol. 32, 1581–1597 (2015).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Darriba, D. et al. ModelTest–NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 37, 291–294 (2020).
Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Kumar, S., Stecher, G., Suleski, M. & Hedges, S. B. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 34, 1812–1819 (2017).
Grasso, T. X. Redefinition, stratigraphy and depositional environments of the Mottville Member (Hamilton Group) in central and eastern New York. Dynamic stratigraphy and depositional environments of the Hamilton Group (middle Devonian) in New York State, Part I. NY State Mus. Bull. 457, 5–31 (1986).
Mergl, M. & Massa, D. Devonian and Lower Carboniferous Brachiopods and Bivalves from Western Libya (Universite Claude Bernard-Lyon I, 1992).
Li, Y. et al. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 8, 1721 (2017).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn (Cold Spring Harbor Laboratory Press, 1989).
Belton, J. M. et al. Hi-C: a comprehensive technique to capture the conformation of genomes. Methods 58, 268–276 (2012).
Zeng, Q. et al. High-quality reannotation of the king scallop genome reveals no ‘gene-rich’ feature and evolution of toxin resistance. Comput. Struct. Biotechnol. J. 19, 4954–4960 (2021).
Li, C. et al. Draft genome of the Peruvian scallop Argopecten purpuratus. GigaScience 7, giy031 (2018).
Liu, X. et al. Draft genomes of two Atlantic bay scallop subspecies Argopecten irradians irradians and A. i. concentricus. Sci. Data 7, 99 (2020).
Ranallo-Benavidez, T. R., Jaron, K. S. & Schatz, M. C. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat. Commun. 11, 1432 (2020).
Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27, 722–736 (2017).
Guan, D. et al. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics 36, 2896–2898 (2020).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 19, e112963 (2014).
Durand, N. C. et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 3, 95–98 (2016).
Dudchenko, O. et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356, 92–95 (2017).
Cantarel, B. L. et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18, 188–196 (2008).
Stanke, M. et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34, W435–W439 (2006).
Korf, I. Gene finding in novel genomes. BMC Bioinform. 5, 59 (2004).
Waterhouse, R. M. et al. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol. Biol. Evol. 35, 543–548 (2018).
Sonnhammer, E. L., Eddy, S. R. & Durbin, R. Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28, 405–420 (1997).
Zhang, L., Bao, Z., Wang, S., Huang, X. & Hu, J. Chromosome rearrangements in Pectinidae (Bivalvia: Pteriomorphia) implied based on chromosomal localization of histone H3 gene in four scallops. Genetica 130, 193–198 (2007).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Van der Auwera, G. A. et al. From FastQ data to high–confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 43, 11.10.1–11.10.33 (2013).
Li, R. et al. FOXL2 and DMRT1L are Yin and Yang genes for determining timing of sex differentiation in the bivalve mollusk Patinopecten yessoensis. Front. Physiol. 9, 1166 (2018).
Shi, S. R., Key, M. E. & Kalra, K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 39, 741–748 (1991).
Darling, A. C., Mau, B., Blattner, F. R. & Perna, N. T. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14, 1394–1403 (2004).
Albertin, C. B. et al. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524, 220–224 (2015).
Ip, J. C.-H. et al. Host–endosymbiont genome integration in a deep-sea chemosymbiotic clam. Mol. Biol. Evol. 38, 502–518 (2021).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980).
Tan, G., Polychronopoulos, D. & Lenhard, B. CNEr: a toolkit for exploring extreme noncoding conservation. PLoS Comput. Biol. 15, e1006940 (2019).
Frith, M. C., Hamada, M. & Horton, P. Parameters for accurate genome alignment. BMC Bioinform. 11, 80 (2010).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Kent, W. J. BLAT—the BLAST-like alignment tool. Genome Res. 12, 656–664 (2002).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP–Seq (MACS). Genome Biol. 9, R137 (2008).
Stark R, & Brown, G. DiffBind: Differential Binding Analysis of ChIP–Seq Peak Data (Bioconductor, 2011).
Sherf, B. A., Navarro, S. L., Hannah, R. & Wood, K. V. Dual-luciferase TM reporter assay: an advanced co-reporter technology integrating firefly and Renilla luciferase assays. Promega Notes 57, 2–9 (1996).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Chen, S. et al. De novo analysis of transcriptome dynamics in the migratory locust during the development of phase traits. PLoS ONE 5, e15633 (2010).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Liu, B.-H. et al. DCGL: an R package for identifying differentially coexpressed genes and links from gene expression microarray data. Bioinformatics 26, 2637–2638 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Wang, Y. P. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49 (2012).
Mokrina, M., Nagasawa, K., Kanamori, M., Natsuike, M. & Osada, M. Seasonal composition of immature germ cells in the Yesso scallop identified by vasa-like gene (my-vlg) and protein expression, with evidence of irregular germ cell differentiation accompanied with a high mortality event. Aquac. Rep. 19, 100613 (2021).
Acknowledgements
This research is part of the ongoing M10K+ genome project proposed by M10K+ Consortium and targets sequencing of 10,000 molluscan genomes. We would like to thank J. C. Perry (University of East Anglia) for providing fruit fly transcriptomic resources and Q. Zhou (Zhejiang University) for providing emu genomic resources. We thank Y. Zhang (South China Sea Institute of Oceanology) for assisting the collection of moon scallop samples. We acknowledge the grant support from National Key Research and Development Project (2018YFD0900200), Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2022QNLM050101-1), National Natural Science Foundation of China (32172967, 32130107), Project of Sanya Yazhouwan Science and Technology City Management Foundation (SKJC-KJ-2019KY01), Key R&D Project of Shandong Province (2020ZLYS10, 2021ZLGX03), China Agriculture Research System of MOF and MARA and Taishan Scholar Project Fund of Shandong Province of China.
Author information
Authors and Affiliations
Contributions
Lingling Zhang, S.W. and Z.B. conceived and designed the study. Lingling Zhang, S.W. and L.B. coordinated and supervised the whole study. W.H., Yuli Li, Q.Z. and T.W. conducted the genome sequencing and assembly. L.L., Lijing Zhang, J.W., T.L., M.Z. and R.L. prepared the libraries for transcriptome sequencing. W.H., S.W., Lingling Zhang, L.B., J.W. and Yuli Li participated in genome and transcriptome analysis. Z.G. and Lijing Zhang performed the sex marker verification. Yajuan Li and Lijing Zhang prepared the libraries for ATAC-seq. L.L. conducted the dual-luciferase reporter assay. H.W. and Lijing Zhang performed the histological analysis, RT–qPCR and immunohistochemistry experiments. Q.X., Q.Z., Y.S., Y.Y. and J.Y. participated in scallop culture and sample collection. Z.B., J.H., X.H., S.L., J.L. and Z.P. participated in discussions and provided suggestions for manuscript improvement. S.W., Lingling Zhang, W.H., L.B. and J.W. did most of the writing with input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Chromosomal architecture and synteny comparison of eight scallop species.
(a) The chromosome architectures of P. yessoensis and 7 other scallop species (their chromosomes coloured with reference to P. yessoensis based on gene correspondence). The red star indicates sex chromosome. (b) Chromosome-wide gene synteny comparison between P. yessoensis and 7 other scallop species.
Extended Data Fig. 2 Identification of scallop sex chromosome and sex-determining gene.
(a, b) Searching for sex-related regions among 19 chromosomes of Patinopecten yessoensis and Chlamys farreri. Circle i, the number distribution of female-related 1-kb bins within a 50-kb window. Such bins show significant female-biased read coverage at the p-value threshold of 0.001. The sex-related region is defined by read coverage depth using a 1-kb bin and the threshold of p-value was set at 0.001. Circle ii, the expression profile of female-biased genes across 19 chromosomes. Circle iii, genome-wide distribution of sexually dimorphic SNP loci. Circle iv, the number distribution of male-related 1-kb bins within a 50-kb window. Circle v, the expression profile of male-biased genes across 19 chromosomes. The sex-related region around FOXL2 is indicated by red circle. (c) Circos plot of gene synteny in 19 chromosome pairs between female and male genome assembly of C. farreri. (d) The histogram shows the normalized coverage for female-specific region around the FOXL2 gene of C. farreri. (e) Structural comparison of sex-linked regions among Z and W contigs of female assembly and Z contig of the male assembly in C. farreri.
Extended Data Fig. 3 Phylogenetic and expression analysis of three putative sex-determining genes.
(a–c) Phylogenetic trees of FOXL2, ZNF226l, CYP3A24l and their belonging gene families. (d) Expression profiles of sex-determining genes in mature ovaries and testes of scallops. Differential analysis using the edgeR test with a Bonferroni correction based on n=3 biologically independent samples. The error bars represent the means ± S.E.M. (e & f) Expression profiles of ZNF226l- and CYP3A24l-residing clades, showing ZNF226l and CYP3A24l are the most female-biased copies (indicated by red names).
Extended Data Fig. 4 Gonad histology and FOXL2 expression during early sex differentiation.
(a) The morphology of gonads of P. yessoensis aged 5 to 11 months. (b) Paraffin sections of female and male gonads. Each experiment was repeated twice independently with similar results. In, intestine; Ct, connective tissue; F, follicle; Fc, follicular cell; Sig, sexually indistinguishable gonium; Og, oogonium; Oc, oocyte; Sg, spermatogonium; Sc, spermatocyte. (c) Temporal expression patterns of FOXL2 measured by RT–qPCR profiling of ovaries and testes of scallops. The earliest differential expression of FOXL2 between sexes occurs at around 7 months of age (based on the one-sided t-test), indicating the initiation of sex differentiation. For each month age, at least 8 samples were assayed and all reactions were conducted in triplicate. The error bars represent the means ± S.E.M. (d) Spatial expression patterns of FOXL2 in ovary and testis by immunohistochemistry. FOXL2 protein primarily locates in the germ cells and follicular cells within the follicles in the ovary (top panel) and testis (bottom panel), confirming its role in sex differentiation. Each experiment was repeated twice independently with similar results.
Extended Data Fig. 5 PCR-based validation of female-specific regions in P. yessoensis.
(a) The gonad morphology for 100 assayed females and males. (b, c) PCR products amplified using two sets of female-specific primer pairs (P11 and P14) for 100 female and male individuals sampled from Dalian (Liaoning, P.R China) and 100 individuals sampled from Yantai (Shandong, P.R China). Additional full-scan images are provided in Source Data Extended Fig. 5. Each PCR was repeated twice independently.
Extended Data Fig. 6 Functional characterization of the FOXL2 enhancer.
Top panel, female-related ATAC peaks in the upstream of FOXL2. Middle panel, plasmid construction from FOXL2-peak1 to FOXL2-peak3 (right to left) used in dual-luciferase assays. Bottom panel, enhancer activities of peaks 1–3 as measured by luciferase assays. The peaks 1–3 showed significant enhancer activity compared to the empty vector pGL3-promoter (based on the two-sided t-test with n=3 biologically independent samples). For boxplot, centre line and box limits represent the median, upper and lower quartiles respectively, whereas whiskers are 1.5x interquartile ranges.
Extended Data Fig. 7 Expression profiling and co-expression network analysis of SBGs during female/male gonad development.
(a) The log-based distribution of male/female expression levels from five gonadal stages across all genes (grey curve) and chr. 15 genes (green curve). (b) The expression patterns of constant female-biased genes, constant male-biased genes and rSBGs across five gonadal development stages. Dash lines show the tendency for female (red) and male (blue) across sex differentiated (D), proliferative (P), growing (G), mature (M) and resting (R) stages. The comparison is based on 1,860 constant female-biased genes, 2,284 constant male-biased genes, and 2,279 rSBGs. For boxplot, centre line and box limits represent the median, upper and lower quartiles respectively, whereas whiskers are 1.5x interquartile ranges. (c) Gene module correspondence between female network (FM1–10) and male network (MM1–8). Notably, the female FM1 module that is significantly enriched with both female-biased genes and rSBGs show the strongest correspondence with the male MM1 module that is significantly enriched with male-biased genes. (d) Network visualization of FM1 (left) and MM1 (right) showing the high intramodular connectivity of rSBGs. Top 30% genes with the highest intramodular connectivity in FM1/MM1 are chosen for network display. Red, blue and purple nodes represent constant female-biased genes, constant male-biased genes and rSBGs, respectively. Node size indicates the intramodular connectivity.
Extended Data Fig. 8 Summary of SBG expression across eight scallop species.
The pie charts show the distribution of SBGs for each scallop species. Histograms show the shared numbers of SBGs by different scallop species. For across-species comparison, the shared genes with consistent female-bias or male-bias are indicated by red and blue bars, whereas those showing the opposite sex-bias patterns are indicated by purple bars.
Extended Data Fig. 9 Sex chromosome-related gene duplication and expressional changes in flatfish, fruit fly and human.
(a–c) Summary of transposed gene duplication rates for autosomes and sex chromosomes in flatfish (ZW-type), fruit fly (XY-type) and human (XY-type), with statistics based on the two-sided Fisher’s exact test. Duplicate gene ratio is calculated by dividing transposed duplicate genes by total genes in specified chromosomes or sex-related regions, that is 0.184 (169/918) for DR and 0.093 (1879/20104) for autosomes in flatfish, 0.024 (48/2000) for DR and 0.017 (179/10737) for autosomes in fruit fly, and 0.034 (28/831) for DR and 0.034 (712/21194) for autosomes in human. (d–f) The Sankey diagrams show the expressional dynamics of duplicated A copies with their parental Z/X copies across various gonad developmental stages of flatfish (48-, 68- and 128-day post hatching), fruit fly (larva, pre-pupa and adult) and human (7, 12 and 17 postconceptional weeks). Pie charts show the proportion of constant SBGs (red or blue) and rSBGs (purple) based on the comparison between Z/X copies and duplicated A copies.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12 and Tables 3–8, 11, 12 and 16.
Supplementary Tables
Supplementary Table 1. Taxon sampling for the phylogeny based on 12S rRNA, 16S rRNA and 28S rRNA. Table 2. Summary of genomic/transcriptomic datasets and accession numbers. Table 9. Distribution of SBGs in adult tissues of P. yessoensis. Table 10. Distribution of SBGs across five gonad developmental stages of P. yessoensis. Table 13. KEGG enrichment analysis of scallop SBGs based on the two-sided chi-square test. Table 14. GO enrichment analysis of scallop SBGs based on the two-sided chi-square test. Table 15. Summary of the co-expression gene networks of scallop. Table 17. Summary of SBGs across eight scallop genomes. Table 18. Summary of sex chromosome-related transposed gene duplication in the DR of emu. Table 19. Summary of sex chromosome-related transposed gene duplication in the DR of chicken. Table 20. Summary of sex chromosome-related transposed gene duplication in the DR of flatfish. Table 21. Summary of sex chromosome-related transposed gene duplication in the DR of fruit fly. Table 22. Summary of sex chromosome-related transposed gene duplication in the DR of human.
Source data
Source Data Fig. 2
Unprocessed gels for Fig. 2d.
Source Data Extended Data Fig. 5
Unprocessed gels for Fig. 5b,c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, W., Liu, L., Wang, J. et al. Ancient homomorphy of molluscan sex chromosomes sustained by reversible sex-biased genes and sex determiner translocation. Nat Ecol Evol 6, 1891–1906 (2022). https://doi.org/10.1038/s41559-022-01898-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01898-6
This article is cited by
-
Genome-wide investigation of the TGF-β superfamily in scallops
BMC Genomics (2024)
-
Oldest known animal sex chromosome evolved in octopuses 380 million years ago
Nature (2024)
-
Evolution and expression patterns of the neo-sex chromosomes of the crested ibis
Nature Communications (2024)
-
Pan-evolutionary and regulatory genome architecture delineated by an integrated macro- and microsynteny approach
Nature Protocols (2024)
-
Multi-omic insights into the formation and evolution of a novel shell microstructure in oysters
BMC Biology (2023)