Abstract
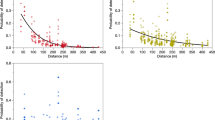
Data suggest that the malaria vector mosquito Anopheles coluzzii persists during the dry season in the Sahel through a dormancy mechanism known as aestivation; however, the contribution of aestivation compared with alternative strategies such as migration is unknown. Here we marked larval Anopheles mosquitoes in two Sahelian villages in Mali using deuterium (2H) to assess the contribution of aestivation to persistence of mosquitoes through the seven-month dry season. After an initial enrichment period, 33% of An. coluzzii mosquitoes were strongly marked. Seven months following enrichment, multiple analysis methods supported the ongoing presence of marked mosquitoes, compatible with the prediction that the fraction of marked mosquitoes should remain stable throughout the dry season if local aestivation is occurring. The results suggest that aestivation is a major persistence mechanism of An. coluzzii in the Sahel, contributing at least 20% of the adults at the onset of rains. This persistence strategy could influence mosquito control and malaria elimination campaigns.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The dataset used in our analyses can be found at https://doi.org/10.6084/m9.figshare.20387328.v1.
Code availability
SAS code associated with these analyses will be made available by T.L. upon request (tlehmann@niaid.nih.gov).
References
Adamou, A. et al. The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar. J. 10, 151 (2011).
Huestis, D. L. et al. Seasonal variation in metabolic rate, flight activity and body size of Anopheles gambiae in the Sahel. J. Exp. Biol. 215, 2013–2021 (2012).
Huestis, D. L. et al. Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J. Exp. Biol. 214, 2345–2353 (2011).
Lehmann, T. et al. Aestivation of the African malaria mosquito, Anopheles gambiae in the Sahel. Am. J. Trop. Med. Hyg. 83, 601–606 (2010).
Yaro, A. S. et al. Dry season reproductive depression of Anopheles gambiae in the Sahel. J. Insect Physiol. 58, 1050–1059 (2012).
Omer, S. M. & Cloudsley-Thompson, J. L. Survival of female Anopheles gambiae Giles through a 9-month dry season in Sudan. Bull. World Health Organ. 42, 319 (1970).
Omer, S. M. & Cloudsley-Thompson, J. L. Dry season biology of Anopheles gambiae Giles in the Sudan. Nature 217, 879–880 (1968).
Holstein, M. H. Biology of Anopheles gambiae (1954). World Health Organization.
Andrade, C. M. et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat. Med. 26, 1929–1940 (2020).
Coulibaly, D. et al. Spatio-temporal dynamics of asymptomatic malaria: bridging the gap between annual malaria resurgences in a Sahelian environment. Am. J. Trop. Med. Hyg. 27, 1761–1769 (2017).
Gillies, M. & Wilkes, T. A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in north-eastern Tanzania. Bull. Entomol. Res. 56, 237–262 (1965).
Gillies, M. T. & De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) (Johannesburg: South African Institute for Medical Research, 1968).
Dao, A. et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature 516, 387–390 (2014).
Thomson, J. G. Malaria in Nyasaland. Proc. R. Soc. Med. 28, 391–404 (1934).
Huestis, D. L. et al. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574, 404–408 (2019).
Lambert, B., North, A., Burt, A. & Godfray, H. C. J. The use of driving endonuclease genes to suppress mosquito vectors of malaria in temporally variable environments. Malar. J. 17, 154 (2018).
Verhulst, N. O., Loonen, J. A. C. M. & Takken, W. Advances in methods for colour marking of mosquitoes. Parasit. Vectors 6, 200 (2013).
Hagler, J. R. & Jackson, C. G. Methods for marking insects: current techniques and future prospects. Annu. Rev. Entomol. 46, 511–543 (2001).
Hamer, G. L. et al. Dispersal of adult culex mosquitoes in an urban West Nile virus hotspot: a mark–capture study incorporating stable isotope enrichment of natural larval habitats. PLoS Negl. Trop. Dis. 8, e2768 (2014).
Hamer, G. L. et al. Evaluation of a stable isotope method to mark naturally-breeding larval mosquitoes for adult dispersal studies. J. Med. Entomol. 49, 61–70 (2012).
Opiyo, M. A. et al. Using stable isotopes of carbon and nitrogen to mark wild populations of Anopheles and Aedes mosquitoes in south-eastern Tanzania. PLoS ONE 11, e0159067 (2016).
Hood-Nowotny, R., Mayr, L. & Knols, B. Use of carbon-13 as a population marker for Anopheles arabiensis in a sterile insect technique (SIT) context. Malar. J. 5, 6 (2006).
Hood-Nowotny, R. & Knols, B. G. J. Stable isotope methods in biological and ecological studies of arthropods. Entomol. Exp. Appl. 124, 3–16 (2007).
Hood-Nowotny, R. et al. Intrinsic and synthetic stable isotope marking of tsetse flies. J. Insect Sci. 11, 79 (2011).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 57, 1758–1784 (2018).
Copia, L., Wassenaar, L. I., Terzer-Wassmuth, S., Belachew, D. L. & Araguas-Araguas, L. J. Comparative evaluation of 2H- versus 3H-based enrichment factor determination on the uncertainty and accuracy of low-level tritium analyses of environmental waters. Appl. Radiat. Isot. 176, 109850 (2021).
Begon, M., Harper, J. & Townsend, C. Ecology: Individuals, Populations and Communities (Blackwell Science, 1996).
Faiman, R. et al. Marking mosquitoes in their natural larval sites using 2H-enriched water: a promising approach for tracking over extended temporal and spatial scales. Methods Ecol. Evol. 10, 1274–1285 (2019).
Florkin, M. Chemical Zoology: Arthropoda Part B (Academic Press, 2014).
Hackman, R. H. & Goldberg, M. Studies on chitin VI. The nature of alpha-and beta-chitins. Aust. J. Biol. Sci. 18, 935–946 (1965).
Faiman, R. et al. Quantifying flight aptitude variation in wild Anopheles gambiae in order to identify long-distance migrants. Malar. J. 19, 263 (2020).
Huestis, D. L. & Lehmann, T. Ecophysiology of Anopheles gambiae s.l.: persistence in the Sahel. Infect. Genet. Evol. 28, 648–661 (2014).
Lehmann, T. et al. Seasonal variation in spatial distributions of Anopheles gambiae in a Sahelian village: evidence for aestivation. J. Med. Entomol. 51, 27–38 (2014).
Costantini, C. et al. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Med. Vet. Entomol. 10, 203–219 (1996).
Toure, Y. T. et al. Mark–release–recapture experiments with Anopheles gambiae s.l. in Banambani Village, Mali, to determine population size and structure. Med. Vet. Entomol. 12, 74–83 (1998).
Faiman, R. et al. A novel fluorescence and DNA combination for versatile, long-term marking of mosquitoes. Methods Ecol. Evol. https://doi.org/10.1111/2041-210X.13592 (2021).
Brattström, O., Bensch, S., Wassenaar, L. I., Hobson, K. A. & Åkesson, S. Understanding the migration ecology of European red admirals Vanessa atalanta using stable hydrogen isotopes. Ecography 33, 720–729 (2010).
Hobson, K. A., Jinguji, H., Ichikawa, Y., Kusack, J. W. & Anderson, R. C. Long-distance migration of the globe skimmer dragonfly to Japan revealed using stable hydrogen (δ 2H) isotopes. Environ. Entomol. 50, 247–255 (2020).
Schilling, E. G. et al. Phenological and isotopic evidence for migration as a life history strategy in Aeshna canadensis (family: Aeshnidae) dragonflies. Ecol. Entomol. 46, 209–219 (2021).
Girard, P., Hillaire-Marcel, C. & Oga, M. S. Determining the recharge mode of Sahelian aquifers using water isotopes. J. Hydrol. 197, 189–202 (1997).
Gutiérrez-Expósito, C., Ramírez, F., Afán, I., Forero, M. & Hobson, K. A. Toward a deuterium feather isoscape for sub-Saharan Africa: progress, challenges and the path ahead. PLoS ONE https://doi.org/10.1371/journal.pone.0135938 (2015).
Lutz, A., Thomas, J. M. & Panorska, A. Environmental controls on stable isotope precipitation values over Mali and Niger, West Africa. Environ. Earth Sci. 62, 1749–1759 (2011).
Risi, C. et al. Understanding the Sahelian water budget through the isotopic composition of water vapor and precipitation. J. Geophys. Res. Atmos. 115, 1–23 (2010).
Tremoy, G. et al. A 1-year long δ18O record of water vapor in Niamey (Niger) reveals insightful atmospheric processes at different timescales. Geophys. Res. Lett. 39, 1–5 (2012).
Terzer‐Wassmuth, S., Wassenaar, L. I., Welker, J. M., Araguás-Araguás, L. J. Improved high‐resolution global and regionalized isoscapes of δ18O, δ2H and d‐excess in precipitation. Hydrol. Process. 35 (2021).
Hobson, K. A. et al. A multi-isotope (δ13C, δ15N, δ2H) feather isoscape to assign Afrotropical migrant birds to origins. Ecosphere 3, art44 (2012).
Diuk-Wasser, M. A. et al. Effect of rice cultivation patterns on malaria vector abundance in rice-growing villages in Mali. Am. J. Trop. Med. Hyg. 76, 869–874 (2007).
Sogoba, N. et al. Malaria transmission dynamics in Niono, Mali: the effect of the irrigation systems. Acta Trop. 101, 232–240 (2007).
Florio, J. et al. Diversity, dynamics, direction, and magnitude of high-altitude migrating insects in the Sahel. Sci. Rep. 10, 20523 (2020).
Wilkins, E. E., Howell, P. I. & Benedict, M. Q. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar. J. 5, 125 (2006).
Wassenaar, L. I. & Hobson, K. A. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ. Health Stud. 39, 211–217 (2003).
Chesson, L. A., Podlesak, D. W., Cerling, T. E. & Ehleringer, J. R. Evaluating uncertainty in the calculation of non-exchangeable hydrogen fractions within organic materials. Rapid Commun. Mass Spectrom. 23, 1275–1280 (2009).
Schimmelmann, A. Determination of the concentration and stable isotopic composition of nonexchangeable hydrogen in organic matter. Anal. Chem. 63, 2456–2459 (1991).
Speakman, J. Doubly Labelled Water: Theory and Practice (Chapman & Hall, 1997).
Base SAS 9.4 Procedures Guide (SAS Institute, 2015).
Cade, B. S. & N, B. R. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 1, 412–420 (2003).
SAS/STAT® 15.1 User’s Guide (SAS Institute, 2018).
Mcclintock, B. T. et al. Uncovering ecological state dynamics with hidden Markov models. Ecol. Lett. 23, 1878–1903 (2020).
Issam, M., Naulet, N., Martin, M. L. & Martin, G. J. A site-specific and multielement approach to the determination of liquid–vapor isotope fractionation parameters: the case of alcohols. J. Phys. Chem. 94, 8303–8309 (1990).
Linderstrøm-Lang, C. U. & Vaslow, F. Isotope effect on the vapor pressures of water–ethanol and deuterium oxide–ethanol-d mixtures. J. Phys. Chem. 72, 2645–2650 (1968).
Ventura, M. & Jeppesen, E. Effects of fixation on freshwater invertebrate carbon and nitrogen isotope composition and its arithmetic correction. Hydrobiologia 632, 297–308 (2009).
Acknowledgements
We thank C. Barillas-Mury, J. Ribeiro, K. A. Hobson, L. I. Wassenaar, G. Hamer, and D. X. Soto for ideation and critical reading of earlier versions of this manuscript. Special thanks is extended to C. A. M. France from The Smithsonian Museum Support Center and J. Matthews of U.C. Davis for help with IRMS work. We thank Dr. T. Wellems for his continuing support of our work. We thank F. Bathily, L. Juompan, M. Sullivan, A. Laughinghouse, K. Lee and S. Moretz for logistic support. We thank the residents of the villages of Thierola and M’Piabougou for their cooperation and hospitality. A special thanks goes to our spouses and families for their endurance, dedication and unwavering support throughout this endeavour. This study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Author information
Authors and Affiliations
Contributions
Conception and study design were by R.F., A.S.Y., A.D. and T.L. Fieldwork and sample collection were done by A.D., A.S.Y., M.D., D.S., Z.L.S. and O.Y. Mosquito identification and sample preparation were handled by R.F., L.M.V., L.C.G., A.R.C. and C.K. Data acquisition (IRMS) was by R.F., A.S.Y., A.D. and T.L. Data interpretation and analysis were done by R.F., A.S.Y., A.D., B.J.K. and T.L. Manuscript drafting was by R.F., A.S.Y. and T.L. Manuscript revision was by R.F., L.M.V., L.C.G., A.R.C., C.K., B.J.K., A.D., A.S.Y., M.D., D.S., Z.L.S., O.Y. and T.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Francesco Baldini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
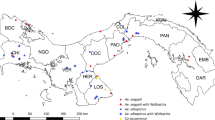
Extended Data Fig. 1 Map of study area.
Map of study area including its location (black rectangle) on a map of Mali (inset). Location of the focal villages (red) surrounded by nearby villages (green) and distant villages (blue) is shown. Maps plotted using SAS 9.4 which is licensed to include maps from Gfk GeoMarketing GmbH (inset) and Open Street map.
Extended Data Fig. 2 Thierola and M’Piabougou satellite images.
Thierola (top) and M’Piabougou (bottom) satellite images; houses (black empty circles) and larval sites (Navy-blue dots). Image data from Google (©2022), Maxar Technologies and CNES/Airbus.
Extended Data Fig. 3 Residual marking.
Differences between pre-enrichment mosquitoes from Thierola collected indoors (September 2017: PreEnrContInd), experimentally marked mosquitoes collected as pupae and stage-4 instar larvae from enriched larval sites during enrichment (LV1Enrich and LV5Enrich), and naturally occurring pupae and stage-4 instar larvae collected after the first rain the following year (July 2018: LV1PostEnrich and LV5PostEnrich). Sample size is shown in blue, and green reference lines show the range of natural 2H levels as in Fig. 2.
Extended Data Fig. 4 Natural temporal variation within and between population in median 2H.
Natural temporal variation within and between population in median 2H (Y axis), spread of 2H as measured by the inter-quartile range (bubble size), and skewness measured by the non-parametric skewness (values inside bubbles; blue denotes negative skewness and yellow and red denote weaker and stronger positive skewness, respectively as seen in scale bar). Sampling years (August to July) are shown on each line and the letters T and M denote samples for Thierola and M’Piabougou.
Supplementary information
Supplementary Information
Supplementary methods and results summary table.
Rights and permissions
About this article
Cite this article
Faiman, R., Yaro, A.S., Dao, A. et al. Isotopic evidence that aestivation allows malaria mosquitoes to persist through the dry season in the Sahel. Nat Ecol Evol 6, 1687–1699 (2022). https://doi.org/10.1038/s41559-022-01886-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01886-w
This article is cited by
-
Potential persistence mechanisms of the major Anopheles gambiae species complex malaria vectors in sub-Saharan Africa: a narrative review
Malaria Journal (2023)
-
Resolving malaria’s dry-season dilemma
Nature Ecology & Evolution (2022)