Abstract
Identifying factors that influence how ectothermic animals respond physiologically to changing temperatures is of high importance given current threats of global climate change. Host-associated microbial communities impact animal physiology and have been shown to influence host thermal tolerance in invertebrate systems. However, the role of commensal microbiota in the thermal tolerance of ectothermic vertebrates is unknown. Here we show that experimentally manipulating the tadpole microbiome through environmental water sterilization reduces the host’s acute thermal tolerance to both heat and cold, alters the thermal sensitivity of locomotor performance, and reduces animal survival under prolonged heat stress. We show that these tadpoles have reduced activities of mitochondrial enzymes and altered metabolic rates compared with tadpoles colonized with unmanipulated microbiota, which could underlie differences in thermal phenotypes. These results demonstrate a strong link between the microbiota of an ectothermic vertebrate and the host’s thermal tolerance, performance and fitness. It may therefore be important to consider host-associated microbial communities when predicting species’ responses to climate change.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw microbiome sequencing data are available from NCBI’s Sequence Read Archive under accession no. PRJNA732310. All other raw datasets are available from the Zenodo repository at https://doi.org/10.5281/zenodo.5703371.
Code availability
The R code used for the statistical analyses in this study is available from the Zenodo repository at https://doi.org/10.5281/zenodo.5703371.
References
Paaijmans, K. P. et al. Temperature variation makes ectotherms more sensitive to climate change. Glob. Change Biol. 19, 2373–2380 (2013).
Clusella-Trullas, S., Blackburn, T. M. & Chown, S. L. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751 (2011).
Pounds, J. A. et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167 (2006).
Sinervo, B. et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 (2010).
Pacifici, M. et al. Assessing species vulnerability to climate change. Nat. Clim. Change 5, 215–224 (2015).
Angilletta, M. J. Jr Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford Univ. Press, 2009).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830 (2011).
Jørgensen, L. B., Malte, H. & Overgaard, J. How to assess Drosophila heat tolerance: unifying static and dynamic tolerance assays to predict heat distribution limits. Funct. Ecol. 33, 629–642 (2019).
Pörtner, H.-O., Bock, C. & Mark, F. C. Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J. Exp. Biol. 220, 2685–2696 (2017).
Gangloff, E. J. & Telemeco, R. S. High temperature, oxygen, and performance: insights from reptiles and amphibians. Integr. Comp. Biol. 58, 9–24 (2018).
Perry, G. M., Danzmann, R. G., Ferguson, M. M. & Gibson, J. P. Quantitative trait loci for upper thermal tolerance in outbred strains of rainbow trout (Oncorhynchus mykiss). Heredity 86, 333–341 (2001).
Healy, T. M. & Schulte, P. M. Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus). J. Comp. Physiol. B 182, 49–62 (2012).
Hu, X. P. & Appel, A. G. Seasonal variation of critical thermal limits and temperature tolerance in Formosan and eastern subterranean termites (Isoptera: Rhinotermitidae). Environ. Entomol. 33, 197–205 (2004).
Nyamukondiwa, C. & Terblanche, J. S. Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): effects of age, gender and feeding status. J. Therm. Biol. 34, 406–414 (2009).
Greenspan, S. E. et al. Infection increases vulnerability to climate change via effects on host thermal tolerance. Sci. Rep. 7, 9349 (2017).
Padfield, D., Castledine, M. & Buckling, A. Temperature-dependent changes to host–parasite interactions alter the thermal performance of a bacterial host. ISME J. 14, 389–398 (2020).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Alberdi, A., Aizpurua, O., Bohmann, K., Zepeda-Mendoza, M. L. & Gilbert, M. T. P. Do vertebrate gut metagenomes confer rapid ecological adaptation? Trends Ecol. Evol. 31, 689–699 (2016).
Kohl, K. D. & Carey, H. V. A place for host–microbe symbiosis in the comparative physiologist’s toolbox. J. Exp. Biol. 219, 3496–3504 (2016).
Fontaine, S. S. & Kohl, K. D. Optimal integration between host physiology and functions of the gut microbiome. Phil. Trans. R. Soc. B 375, 20190594 (2020).
Velagapudi, V. R. et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 51, 1101–1112 (2010).
Donohoe, D. R. et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 (2011).
Ziegler, M., Seneca, F. O., Yum, L. K., Palumbi, S. R. & Voolstra, C. R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 8, 14213 (2017).
Russell, J. A. & Moran, N. A. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. R. Soc. B 273, 603–610 (2006).
Montllor, C. B., Maxmen, A. & Purcell, A. H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27, 189–195 (2002).
Herrera, M. et al. Unfamiliar partnerships limit cnidarian holobiont acclimation to warming. Glob. Change Biol. 26, 5539–5553 (2020).
Jaramillo, A. & Castaneda, L. E. Gut microbiota of Drosophila subobscura contributes to its heat tolerance and is sensitive to transient thermal stress. Front. Microbiol. 12, 886 (2021).
Moghadam, N. N. et al. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly 12, 1–12 (2018).
Fontaine, S. S., Novarro, A. J. & Kohl, K. D. Environmental temperature alters the digestive performance and gut microbiota of a terrestrial amphibian. J. Exp. Biol. 221, 187559 (2018).
Kohl, K. D. & Yahn, J. Effects of environmental temperature on the gut microbial communities of tadpoles. Environ. Microbiol. 18, 1561–1565 (2016).
Fontaine, S. S. & Kohl, K. D. The gut microbiota of invasive bullfrog tadpoles responds more rapidly to temperature than a non‐invasive congener. Mol. Ecol. 29, 2449–2462 (2020).
Bestion, E. et al. Climate warming reduces gut microbiota diversity in a vertebrate ectotherm. Nat. Ecol. Evol. 1, 0161 (2017).
Zhu, L. et al. Environmental temperatures affect the gastrointestinal microbes of the Chinese giant salamander. Front. Microbiol. 12, 493 (2021).
Moeller, A. H. et al. The lizard gut microbiome changes with temperature and is associated with heat tolerance. Appl. Environ. Microbiol. 86, e01181-20 (2020).
Kokou, F. et al. Host genetic selection for cold tolerance shapes microbiome composition and modulates its response to temperature. eLife 7, e36398 (2018).
Hanage, W. P. Microbiology: microbiome science needs a healthy dose of scepticism. Nature 512, 247–248 (2014).
Pascoe, E. L., Hauffe, H. C., Marchesi, J. R. & Perkins, S. E. Network analysis of gut microbiota literature: an overview of the research landscape in non-human animal studies. ISME J. 11, 2644–2651 (2017).
Mykles, D. L., Ghalambor, C. K., Stillman, J. H. & Tomanek, L. Grand challenges in comparative physiology: integration across disciplines and across levels of biological organization. Integr. Comp. Biol. 50, 6–16 (2010).
Kohl, K. D. A microbial perspective on the grand challenges in comparative animal physiology. mSystems 3, e00146-17 (2018).
Gray, K. T., Escobar, A. M., Schaeffer, P. J., Mineo, P. M. & Berner, N. J. Thermal acclimatization in overwintering tadpoles of the green frog, Lithobates clamitans (Latreille, 1801). J. Exp. Zool. A 325, 285–293 (2016).
Brattstrom, B. H. & Lawrence, P. The rate of thermal acclimation in anuran amphibians. Physiol. Zool. 35, 148–156 (1962).
Knutie, S. A., Wilkinson, C. L., Kohl, K. D. & Rohr, J. R. Early-life disruption of amphibian microbiota decreases later-life resistance to parasites. Nat. Commun. 8, 86 (2017).
Warne, R. W., Kirschman, L. & Zeglin, L. Manipulation of gut microbiota during critical developmental windows affects host physiological performance and disease susceptibility across ontogeny. J. Anim. Ecol. 88, 845–856 (2019).
Morgun, A. et al. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut 64, 1732–1743 (2015).
Kohl, K. D., Cary, T. L., Karasov, W. H. & Dearing, M. D. Restructuring of the amphibian gut microbiota through metamorphosis. Environ. Microbiol. Rep. 5, 899–903 (2013).
Vences, M. et al. Gut bacterial communities across tadpole ecomorphs in two diverse tropical anuran faunas. Sci. Nat. 103, 25 (2016).
Fontaine, S. S., Mineo, P. M. & Kohl, K. D. Changes in the gut microbial community of the eastern newt (Notophthalmus viridescens) across its three distinct life stages. FEMS Microbiol. Ecol. 97, fiab021 (2021).
Anderson, M. J. & Walsh, D. C. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol. Monogr. 83, 557–574 (2013).
Sepulveda, J. & Moeller, A. H. The effects of temperature on animal gut microbiomes. Front. Microbiol. 11, 384 (2020).
Arango, R. A., Schoville, S. D., Currie, C. R. & Carlos-Shanley, C. Experimental warming reduces survival, cold tolerance, and gut prokaryotic diversity of the eastern subterranean termite, Reticulitermes flavipes (Kollar). Front. Microbiol. 12, 1116 (2021).
Stothart, M. R. et al. Stress and the microbiome: linking glucocorticoids to bacterial community dynamics in wild red squirrels. Biol. Lett. 12, 20150875 (2016).
Zaneveld, J. R., McMinds, R. & Thurber, R. V. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2, 17121 (2017).
Orrock, J. L. & Watling, J. I. Local community size mediates ecological drift and competition in metacommunities. Proc. R. Soc. B 277, 2185–2191 (2010).
Deeg, C. M. et al. Chromulinavorax destructans, a pathogen of microzooplankton that provides a window into the enigmatic candidate phylum Dependentiae. PLoS Pathog. 15, e1007801 (2019).
Kaboré, O. D., Godreuil, S. & Drancourt, M. Planctomycetes as host-associated bacteria: a perspective that holds promise for their future isolations, by mimicking their native environmental niches in clinical microbiology laboratories. Front. Cell. Infect. Microbiol. 10, 729 (2020).
Sheremet, A. et al. Ecological and genomic analyses of candidate phylum WPS‐2 bacteria in an unvegetated soil. Environ. Microbiol. 22, 3143–3157 (2020).
Correa, D. T. et al. Multilevel community assembly of the tadpole gut microbiome. Preprint at bioRxiv https://doi.org/10.1101/2020.07.05.188698 (2020).
Contijoch, E. J. et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. eLife 8, e40553 (2019).
Warne, R. W., Kirschman, L. & Zeglin, L. Manipulation of gut microbiota reveals shifting community structure shaped by host developmental windows in amphibian larvae. Integr. Comp. Biol. 57, 786–794 (2017).
Trevelline, B. K., Fontaine, S. S., Hartup, B. K. & Kohl, K. D. Conservation biology needs a microbial renaissance: a call for the consideration of host-associated microbiota in wildlife management practices. Proc. R. Soc. B 286, 20182448 (2019).
Lutterschmidt, W. I. & Hutchison, V. H. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574 (1997).
Gosner, K. L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 (1960).
Daloso, D. M. The ecological context of bilateral symmetry of organ and organisms. Nat. Sci. 6, 43340 (2014).
Goldstein, J. A., Hoff, K. v. S. & Hillyard, S. D. The effect of temperature on development and behaviour of relict leopard frog tadpoles. Conserv. Physiol. 5, cow075 (2017).
Harkey, G. A. & Semlitsch, R. D. Effects of temperature on growth, development, and color polymorphism in the ornate chorus frog Pseudacris ornata. Copeia 1998, 1001–1007 (1988).
Marian, M. & Pandian, T. Effect of temperature on development, growth and bioenergetics of the bullfrog tadpole Rana tigrina. J. Therm. Biol. 10, 157–161 (1985).
Alvarez, D. & Nicieza, A. Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct. Ecol. 16, 640–648 (2002).
Kohl, K. D., Brun, A., Bordenstein, S. R., Caviedes‐Vidal, E. & Karasov, W. H. Gut microbes limit growth in house sparrow nestlings (Passer domesticus) but not through limitations in digestive capacity. Integr. Zool. 13, 139–151 (2018).
Potti, J. et al. Bacteria divert resources from growth for Magellanic penguin chicks. Ecol. Lett. 5, 709–714 (2002).
Coates, M. E., Fuller, R., Harrison, G., Lev, M. & Suffolk, S. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. Br. J. Nutr. 17, 141–150 (1963).
Gaskins, H., Collier, C. & Anderson, D. Antibiotics as growth promotants: mode of action. Anim. Biotechnol. 13, 29–42 (2002).
Gitsels, A., Sanders, N. & Vanrompay, D. Chlamydial infection from outside to inside. Front. Microbiol. 10, 2329 (2019).
Denver, R. J. Proximate mechanisms of phenotypic plasticity in amphibian metamorphosis. Am. Zool. 37, 172–184 (1997).
Chevalier, C. et al. Gut microbiota orchestrates energy homeostasis during cold. Cell 163, 1360–1374 (2015).
Khakisahneh, S., Zhang, X.-Y., Nouri, Z. & Wang, D.-H. Gut microbiota and host thermoregulation in response to ambient temperature fluctuations. mSystems 5, e00514–e00520 (2020).
Xie, B. et al. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 7, 1544–1555 (2013).
Gutiérrez‐Pesquera, L. M. et al. Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. J. Biogeogr. 43, 1166–1178 (2016).
Litmer, A. R. & Murray, C. M. Critical thermal tolerance of invasion: comparative niche breadth of two invasive lizards. J. Therm. Biol. 86, 102432 (2019).
Semlitsch, R. D. Effects of body size, sibship, and tail injury on the susceptibility of tadpoles to dragonfly predation. Can. J. Zool. 68, 1027–1030 (1990).
Cabrera-Guzmán, E., Crossland, M. R., Brown, G. P. & Shine, R. Larger body size at metamorphosis enhances survival, growth and performance of young cane toads (Rhinella marina). PLoS ONE 8, e70121 (2013).
Tejedo, M. Effects of body size and timing of reproduction on reproductive success in female natterjack toads (Bufo calamita). J. Zool. 228, 545–555 (1992).
Warne, R. W., Crespi, E. J. & Brunner, J. L. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol. 25, 139–146 (2011).
Urban, M. C. Accelerating extinction risk from climate change. Science 348, 571–573 (2015).
Pearce, T. A. & Paustian, M. E. Are temperate land snails susceptible to climate change through reduced altitudinal ranges? A Pennsylvania example. Am. Malacol. 31, 213–224 (2013).
Wolfe, D. W. et al. Projected change in climate thresholds in the northeastern US: implications for crops, pests, livestock, and farmers. Mitig. Adapt. Strateg. Glob. Change 13, 555–575 (2008).
Huey, R. B. & Kingsolver, J. G. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 4, 131–135 (1989).
Bennett, A. F. Thermal dependence of locomotor capacity. Am. J. Physiol. 259, R253–R258 (1990).
Seebacher, F. & Walter, I. Differences in locomotor performance between individuals: importance of parvalbumin, calcium handling and metabolism. J. Exp. Biol. 215, 663–670 (2012).
Husak, J. F., Fox, S. F., Lovern, M. B. & Bussche, R. A. V. D. Faster lizards sire more offspring: sexual selection on whole‐animal performance. Evolution 60, 2122–2130 (2006).
Mineo, P. M., Waldrup, C., Berner, N. J. & Schaeffer, P. J. Differential plasticity of membrane fatty acids in northern and southern populations of the eastern newt (Notophthalmus viridescens). J. Comp. Physiol. B 189, 249–260 (2019).
Chung, D. J., Sparagna, G. C., Chicco, A. J. & Schulte, P. M. Patterns of mitochondrial membrane remodeling parallel functional adaptations to thermal stress. J. Exp. Biol. 221, 174458 (2018).
Gladwell, R., Bowler, K. & Duncan, C. Heat death in crayfish Austropotamobius pallipes: ion movements and their effects on excitable tissues during heat death. J. Therm. Biol. 1, 79–94 (1976).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Pörtner, H. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146 (2001).
Gräns, A. et al. Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J. Exp. Biol. 217, 711–717 (2014).
Jutfelt, F. et al. Oxygen- and capacity-limited thermal tolerance: blurring ecology and physiology. J. Exp. Biol. 221, 169615 (2018).
St-Pierre, J., Charest, P.-M. & Guderley, H. Relative contribution of quantitative and qualitative changes in mitochondria to metabolic compensation during seasonal acclimatisation of rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 201, 2961–2970 (1998).
Grim, J., Miles, D. & Crockett, E. Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J. Exp. Biol. 213, 445–452 (2010).
LeMoine, C. M., Genge, C. E. & Moyes, C. D. Role of the PGC-1 family in the metabolic adaptation of goldfish to diet and temperature. J. Exp. Biol. 211, 1448–1455 (2008).
McClelland, G. B., Craig, P. M., Dhekney, K. & Dipardo, S. Temperature‐ and exercise‐induced gene expression and metabolic enzyme changes in skeletal muscle of adult zebrafish (Danio rerio). J. Physiol. 577, 739–751 (2006).
Pichaud, N. et al. Cardiac mitochondrial plasticity and thermal sensitivity in a fish inhabiting an artificially heated ecosystem. Sci. Rep. 9, 17832 (2019).
Seebacher, F., Guderley, H., Elsey, R. M. & Trosclair, P. L. Seasonal acclimatisation of muscle metabolic enzymes in a reptile (Alligator mississippiensis). J. Exp. Biol. 206, 1193–1200 (2003).
Berner, N. J. & Bessay, E. P. Correlation of seasonal acclimatization in metabolic enzyme activity with preferred body temperature in the eastern red spotted newt (Notophthalmus viridescens viridescens). Comp. Biochem. Physiol. A 144, 429–436 (2006).
Vigelsø, A., Andersen, N. B. & Dela, F. The relationship between skeletal muscle mitochondrial citrate synthase activity and whole body oxygen uptake adaptations in response to exercise training. Int. J. Physiol. Pathophysiol. Pharmacol. 6, 84–101 (2014).
Li, Y., Park, J.-S., Deng, J.-H. & Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 38, 283–291 (2006).
Pryor, G. S. & Bjorndal, K. A. Symbiotic fermentation, digesta passage, and gastrointestinal morphology in bullfrog tadpoles (Rana catesbeiana). Physiol. Biochem. Zool. 78, 201–215 (2005).
Clark, A. & Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 8, 319 (2017).
Payne, N. L. et al. Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Funct. Ecol. 30, 903–912 (2016).
Van Dijk, P., Tesch, C., Hardewig, I. & Portner, H. Physiological disturbances at critically high temperatures: a comparison between stenothermal Antarctic and eurythermal temperate eelpouts (Zoarcidae). J. Exp. Biol. 202, 3611–3621 (1999).
Schulte, P. M. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866 (2015).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Hoppeler, H. & Weibel, E. R. Scaling functions to body size: theories and facts. J. Exp. Biol. 208, 1573–1574 (2005).
Hopkins, W. A., Rowe, C. L. & Congdon, J. D. Elevated trace element concentrations and standard metabolic rate in banded water snakes (Nerodia fasciata) exposed to coal combustion wastes. Environ. Toxicol. Chem. 18, 1258–1263 (1999).
Sokolova, I. Bioenergetics in environmental adaptation and stress tolerance of aquatic ectotherms: linking physiology and ecology in a multi-stressor landscape. J. Exp. Biol. 224, 236802 (2021).
Sokolova, I. M. & Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim. Res. 37, 181–201 (2008).
Peralta-Maraver, I. & Rezende, E. L. Heat tolerance in ectotherms scales predictably with body size. Nat. Clim. Change 11, 58–63 (2021).
Bahrndorff, S., Alemu, T., Alemneh, T. & Lund Nielsen, J. The microbiome of animals: implications for conservation biology. Int. J. Genomics 2016, 5304028 (2016).
Hauffe, H. C. & Barelli, C. Conserve the germs: the gut microbiota and adaptive potential. Conserv. Genet. 20, 19–27 (2019).
Jiménez, R. R. & Sommer, S. The amphibian microbiome: natural range of variation, pathogenic dysbiosis, and role in conservation. Biodivers. Conserv. 26, 763–786 (2017).
Swaddle, J. P. Fluctuating asymmetry, animal behavior, and evolution. Adv. Study Behav. 32, 169–205 (2003).
R Core Team R: A Language and Environment for Statistical Computing v.3.4.3 (R Foundation for Statistical Computing, 2019).
Bates, D., Machler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. Preprint at https://arxiv.org/abs/1406.5823 (2014).
Pinheiro, J. et al. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3 (2017).
Hulbert, A., Pamplona, R., Buffenstein, R. & Buttemer, W. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 87, 1175–1213 (2007).
Oksanen, J. et al. vegan: Community Ecology Package. R package version 2 (2013).
Mary-Huard, T., Daudin, J.-J., Baccini, M., Biggeri, A. & Bar-Hen, A. Biases induced by pooling samples in microarray experiments. Bioinformatics 23, i313–i318 (2007).
Singer, J. D. & Willett, J. B. It’s about time: using discrete-time survival analysis to study duration and the timing of events. J. Educ. Stat. 18, 155–195 (1993).
Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17, e100442 (2021).
Acknowledgements
We thank M. Ohmer, K. Altman and E. Le Sage for field collection assistance; K. Kohler, M. Maurer, M. Maier, S. Reilly, A. Haid, C. Duckworth and J. Adams for animal husbandry and DNA extraction assistance; and N. Barts for technical assistance. We also thank the University of Pittsburgh’s Health Sciences Metabolomics and Lipidomics core facility (NIH S10OD023402 PI Wendell), the DNA Services Facility at the University of Illinois at Chicago, and the Microbial Analysis, Resources, and Services Facility at the University of Connecticut for sample processing. This work was supported by the University of Pittsburgh (start-up funds to K.D.K.), Elmhurst University (faculty research grant to P.M.M.) and the National Science Foundation (GRFP to S.S.F.).
Author information
Authors and Affiliations
Contributions
S.S.F., K.D.K. and P.M.M. designed the study. S.S.F. and P.M.M. collected the data. S.S.F. analysed the data, generated the figures and wrote the initial manuscript draft with editing from K.D.K. and P.M.M. K.D.K. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Camila Carlos-Shanley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
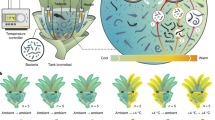
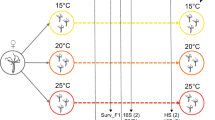
Extended Data Fig. 1 Experimental designs and sample sizes for experiments 1 and 2.
For both experiments, tadpoles were reared from pairs of adult frogs in the laboratory and divided into colonized (C) and depleted (D) water treatments at Gosner stage (GS) 25. For experiment 1, green frog tadpoles and pond water from Louisiana (LA, USA) were used and for experiment 2, green frog tadpoles and pond water from Pennsylvania (PA, USA) were used. Images created with BioRender.com.
Extended Data Fig. 2 Environmental microbial communities of pond water used for the colonized microbial treatment.
a, Principal Coordinate (PCo) analysis plot based on Bray-Curtis dissimilarity between microbial communities of water samples collected fresh from the pond or after storage in the laboratory at 4 °C. b, Principal Coordinate (PCo) analysis plot based on Bray-Curtis dissimilarity between tadpole gut microbial communities and microbial communities of pond water collected fresh from the pond or after storage in the laboratory. Due to overlap between water types on the plot, fresh pond water samples are outlined in black. For both PCoA plots, percentages represent the proportion of variation explained by each axis. c, mean relative abundances of bacterial phyla found in pond water samples fresh from the pond or after storage in the laboratory. The top ten most abundant phyla are shown individually, and the remainder are grouped together as “other”. Any bacteria that were unable to be assigned to a phylum are grouped together as “unassigned”. d, mean relative abundances of bacterial genera found in pond water samples fresh from the pond or after storage in the laboratory. The top ten most abundant genera from each group are shown individually, and the remainder are grouped together as “other”. Any bacteria that were unable to be assigned to a genus are grouped together as “unassigned”. For all figures, N = 2 for fresh pond samples and N = 7 for stored samples.
Extended Data Fig. 3 Mean relative abundances of bacterial phyla in gut microbial communities of tadpoles from experiment 1 across microbial colonization and acclimation temperature treatment groups.
The top ten most abundant phyla are shown individually, and the remainder are grouped together as “other”. Any bacteria that were unable to be assigned to a phylum are grouped together as “unassigned”. N = 27 animals per group.
Extended Data Fig. 4 Impacts of microbial colonization treatment and acclimation temperature on tadpole gut microbial communities in experiment 1.
a, Faith’s phylogenetic diversity of the gut bacterial community b, Shannon diversity of the gut bacterial community c, Pielou’s evenness within the gut bacterial community d, Number of bacterial cells in tadpole gut contents measured using flow cytometry and shown on a log scale e, Principal Coordinate (PCo) analysis plot based on Unweighted UniFrac distance between gut bacterial community samples f, Principal Coordinate analysis (PCo) plot based on Weighted UniFrac distance between gut bacterial community samples. For boxplots a-c, N = 25 for the 14 °C colonized and depleted groups, 26 for the 22 °C colonized and depleted groups, 26 for the 28 °C depleted group, and 27 for the 28 °C colonized group. For boxplot d, N = 3 for the 14 °C colonized group, 10 for the 14 °C depleted group, 10 for the 22 °C colonized group, 11 for the 22 °C depleted group, 10 for the 28 °C depleted group, and 9 for the 28 °C colonized group. For all boxplots, the center line represents the median, the length of the box extends through the IQR, and whiskers extend to 1.5x IQR. All points outside this range are plotted individually. For all principal coordinate analysis plots, percentages represent the proportion of variation explained by each axis. C = colonized tadpoles and D = depleted tadpoles. Colors represent tadpole acclimation temperature.
Extended Data Fig. 5 Plots used to determine gating parameters for flow cytometry from one representative sample.
a, A plot of fluorescein isothiocyanate (FITC) vs. cell counts was used to distinguish cells stained with SYBR Green dye from all other cells b, A plot of forward scatter (FSC) vs. side scatter (SSC) was used to distinguish populations of counting beads from all other events. To establish initial gates for counting beads, blank samples spiked only with beads were used. On both plots, red rectangles represent the gates and surround the events counted. Percentages indicate the proportion of events within the gate out of total events. R1 = stained bacterial cells and R2 = counting beads.
Extended Data Fig. 6 Tadpole morphometrics across microbial colonization and acclimation temperature treatment groups from experiment 1.
a, tadpole body length b, tadpole body width c, tadpole facial symmetry, calculated as the absolute value, subtracted from 1, of the difference between the distance from the center of each eye to the tip of the nose. The center line of each boxplot represents the median, the length of the box extends through the IQR, and whiskers extend to 1.5x IQR. All points outside this range are plotted individually. C = colonized tadpoles and D = depleted tadpoles. Colors represent tadpole acclimation temperature. N = 27 animals per group.
Extended Data Fig. 7 Impact of microbial colonization treatment on tadpole gut microbial communities in experiment 2.
a, Number of bacterial ASVs in the tadpole gut bacterial community, b, Faith’s phylogenetic diversity of the tadpole gut bacterial community, c, Pielou’s evenness of the tadpole gut bacterial community, and d, Principal Coordinate (PCo) analysis plot based on Bray-Curtis dissimilarity between gut bacterial community samples. Percentages represent the proportion of variation explained by each axis. The center line of each boxplot represents the median, the length of the box extends through the IQR, and whiskers extend to 1.5x IQR. All points outside this range are plotted individually. C = colonized tadpoles and D = depleted tadpoles. N = 13 colonized animals and 15 depleted animals.
Extended Data Fig. 8 The impact of microbial colonization treatment on tadpole morphometrics and heat tolerance in experiment 2.
a, tadpole body mass b, tadpole body length c, tadpole developmental stage based on the Gosner system d, tadpole critical thermal maximum (CTmax). The center line of each boxplot represents the median, the length of the box extends through the IQR, and whiskers extend to 1.5x IQR. All points outside this range are plotted individually. C = colonized tadpoles and D = depleted tadpoles. For boxplots a and b, N = 19 colonized animals and 21 depleted animals. For boxplot c, N = 19 colonized animals and 20 depleted animals. For boxplot d, N = 17 colonized animals and 18 depleted animals.
Extended Data Fig. 9 Impacts of microbial colonization treatment and assay temperature on tadpole mass-specific resting metabolic rate.
The center line of each boxplot represents the median, the length of the box extends through the IQR, and whiskers extend to 1.5x IQR. All points outside this range are plotted individually. C = colonized tadpoles and D = depleted tadpoles. On the y-axis VO2 refers to oxygen consumption. N = 10 colonized animals per temperature and 9 depleted animals per temperature.
Supplementary information
Supplementary Information
Supplementary Discussions 1–3, Tables 1–4, Methods and References.
Rights and permissions
About this article
Cite this article
Fontaine, S.S., Mineo, P.M. & Kohl, K.D. Experimental manipulation of microbiota reduces host thermal tolerance and fitness under heat stress in a vertebrate ectotherm. Nat Ecol Evol 6, 405–417 (2022). https://doi.org/10.1038/s41559-022-01686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01686-2