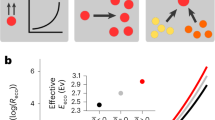
Abstract
Temperature is one of the fundamental environmental variables that determine the composition and function of microbial communities. However, a predictive understanding of how microbial communities respond to changes in temperature is lacking, partly because it is not obvious which aspects of microbial physiology determine whether a species could benefit from a change in the temperature. Here we incorporate how microbial growth rates change with temperature into a modified Lotka–Volterra competition model and predict that higher temperatures should—in general—favour the slower-growing species in a bacterial community. We experimentally confirm this prediction in pairwise cocultures assembled from a diverse set of species and show that these changes to pairwise outcomes with temperature are also predictive of changing outcomes in three-species communities, suggesting that our theory may be applicable to more-complex assemblages. Our results demonstrate that it is possible to predict how bacterial communities will shift with temperature knowing only the growth rates of the community members. These results provide a testable hypothesis for future studies of more-complex natural communities and we hope that this work will help to bridge the gap between ecological theory and the complex dynamics observed in metagenomic surveys.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data are publically available through the FigShare digital repository22.
Code availability
All code for data analysis is available from the first author by request.
References
Gilbert, J. A. et al. Defining seasonal marine microbial community dynamics. ISME J. 6, 298–308 (2012).
Fuhrman, J. A., Cram, J. A. & Needham, D. M. Marine microbial community dynamics and their ecological interpretation. Nat. Rev. Microbiol. 13, 133–146 (2015).
Ward, C. S. et al. Annual community patterns are driven by seasonal switching between closely related marine bacteria. ISME J. 11, 1412–1422 (2017).
Fuhrman, J. A. et al. A latitudinal diversity gradient in planktonic marine bacteria. Proc. Natl Acad. Sci. USA 105, 7774–7778 (2008).
Barton, A. D., Irwin, A. J., Finkel, Z. V. & Stock, C. A. Anthropogenic climate change drives shift and shuffle in North Atlantic phytoplankton communities. Proc. Natl Acad. Sci. USA 113, 2964–2969 (2016).
Deslippe, J. R., Hartmann, M., Simard, S. W. & Mohn, W. W. Long-term warming alters the composition of Arctic soil microbial communities. FEMS Microbiol. Ecol. 82, 303–315 (2012).
Luo, C. et al. Soil microbial community responses to a decade of warming as revealed by comparative metagenomics. Appl. Environ. Microbiol. 80, 1777–1786 (2014).
Jiang, L. & Morin, P. J. Temperature fluctuation facilitates coexistence of competing species in experimental microbial communities. J. Anim. Ecol. 76, 660–668 (2007).
Descamps-Julien, B. & Gonzalez, A. Stable coexistence in a fluctuating environment: an experimental demonstration. Ecology 86, 2815–2824 (2005).
Abreu, C. I., Friedman, J., Andersen Woltz, V. L. & Gore, J. Mortality causes universal changes in microbial community composition. Nat. Commun. 10, 2120 (2019).
Ratkowsky, D. A., Olley, J., McMeekin, T. A. & Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 149, 1–5 (1982).
Rosso, L., Lobry, J. R. & Flandrois, J. P. An unexpected correlation between cardinal temperatures of microbial growth highlighted by a new model. J. Theor. Biol. 162, 447–463 (1993).
Friedman, J., Higgins, L. M. & Gore, J. Community structure follows simple assembly rules in microbial microcosms. Nat. Ecol. Evol. 1, 0109 (2017).
Stubbendieck, R. M. & Straight, P. D. Multifaceted interfaces of bacterial competition. J. Bacteriol. 198, 2145–2155 (2016).
Ratzke, C. & Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 16, e2004248 (2018).
De Carvalho, C. C. R. & Fernandes, P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs 8, 705–727 (2010).
James, P. D. A., Edwards, C. & Dawson, M. The effects of temperature, pH and growth rate on secondary metabolism in Streptomyces thermoviolaceus grown in a chemostat. J. Gen. Microbiol. 137, 1715–1720 (1991).
Sun, W., Qian, X., Gu, J., Wang, X.-J. & Duan, M.-L. Mechanism and effect of temperature on variations in antibiotic resistance genes during anaerobic digestion of dairy manure. Sci. Rep. 6, 30237 (2016).
Kim, C., Wilkins, K., Bowers, M., Wynn, C. & Ndegwa, E. Influence of pH and temperature on growth characteristics of leading foodborne pathogens in a laboratory medium and select food beverages. Austin Food Sci. 3, 1031 (2018).
Lewington-Pearce, L. et al. Temperature-dependence of minimum resource requirements alters competitive hierarchies in phytoplankton. Oikos 128, 1194–1205 (2019).
Hanke, A. et al. Selective pressure of temperature on competition and cross-feeding within denitrifying and fermentative microbial communities. Front. Microbiol. 6, 1461 (2016).
Lax, S., Abreu, C. I. & Gore, J. Higher temperatures generically favor slower-growing bacterial species in multispecies communities. Figshare https://doi.org/10.6084/m9.figshare.8285543.v1 (2020).
Acknowledgements
We thank A. Ortiz for providing us with the bacterial soil isolates and the members of the Gore laboratory for their suggestions and discussion.
Author information
Authors and Affiliations
Contributions
All authors designed the study. S.L. carried out the experiments with assistance from C.I.A. S.L. analysed the data. C.I.A. analysed the Lotka–Volterra and other models, and wrote the Supplementary Information. S.L. wrote the manuscript, and all authors edited and approved it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Predicted phase spaces of coculture outcomes with noncompetitive interactions.
The model is based on a hypothetical faster-growing species with the parameters b = 0.0230 and T0 = −5.7 and slower growing species with b = 0.0250 and T0 = −4.1. In cases where there are multiple non-trivial qualitative outcomes, the specific α’s are indicated at the bottom left of the plot. If no specific α’s are indicated, the qualitative phase space is identical for all α’s in the title range. In mutualist pairs, coexistence is always expected in any region of phase space where the slower-growing species is able to survive the imposed death rate, such that decreasing temperature can only move the outcome into a trivial fast-grower victory (rs < δ < rf) or community collapse. Parasitic interactions, however, depend on the α’s. If the fast grower assists the slow grower (αsf < 0) but the slow grower harms the fast grower (αfs > 0), we expect coexistence in all non-trivial regions of phase space (rs > δ) if αfs < 1 and a transition from coexistence to slow-grower dominance with increasing temperature if αfs > 1. If the slow grower assists the fast grower (αfs < 0) but is hindered by the fast grower (αsf > 0), we expect either consistent fast grower dominance if αsf > 1 or a transition from fast-grower dominance to coexistence with increasing temperature if 0 < αsf < 1.
Extended Data Fig. 2 Fits of the Ratkowsky model to the 13 bacterial strains in this study.
The Ratkowsky model predicts a linear relationship between temperature and the square root of the growth rate. We used linear regression (light green line) on our growth rate estimates below 30 °C (dark green circles, SEM indicated by bars) to estimate b (the slope of the regression) and T0 (the x-intercept of the regression). The 30 °C growth rates ± SEM are plotted in red. The R2 of the regression is also provided.
Extended Data Fig. 3 Phase diagrams of the interaction between temperature and dilution factor for two sets of coculture outcomes.
(a) We varied temperature (4 levels) and dilution factor (6 levels) in a factorial design to understand how these two variables interact to shape competitive landscapes. Here, we have the qualitative outcomes for the Aci1/Pan1 competition for each combination of variables. In cases of coexistence, the percentage of the final community comprised by the slow-grower is indicated. (b) Phase diagram of the model predictions for the Aci1/Pan1 competition, parameterized with the measured b and T0 values, and with α’s (indicated at top left) estimated to best match the experimental outcome. (c, d): As in a, b, but for the PP/PCH competition.
Extended Data Fig. 4 Competitions between a phylogenetically diverse set of species in a complex media further validate the prediction that higher temperatures favor slower-growing bacterial species.
(a) Growth rate measurements for the 6 bacterial species in 100% LB media with an oxygen permeable cover. Error bars represent the standard error of the mean. (b) Equilibrium species fraction after 7 cycles of a 1/100 daily dilution. We competed the 8 pairs of species where there is a consistent fast grower across the range of experimental temperatures. The y-axis represents the fraction of the faster growing species. Competitions were started at two initial conditions: 90% fast grower and 10% fast grower. Asterisks represent the outcome of an individual condition and circles represent the average outcome across the two initial conditions. Cases where the outcome is bistable are indicated in yellow. (c) As in b except with a 1/1000 daily dilution. Note that in both b and c we were unable to count individual colonies in the Aci1/Pan1 competition because of very high cell density. Aci1 and Pan1 always coexisted at approximately equal fractions.
Extended Data Fig. 6 Relationship between coculture outcome and difference in growth rate parameters between the faster-grower and slower-grower.
Correlation values are Pearson correlation, error bars on the y-axis correspond to the SEM, and trend lines are a linear regression with the 95% confidence interval. (a) Correlation between growth rate difference and coculture outcome by temperature. (b) Correlation between T0 difference and coculture outcome by temperature.
Extended Data Fig. 7 Maximal growth rates for 11 species growing in the spent media of the 8 most common species in our set of competition experiments.
Black lines indicate the median, lower and upper box boundaries correspond to the first and third quartiles, and whiskers extend to the largest and smallest values within 1.5 times the inter-quartile range. Colors indicate the origin of the spent media, and black coloration indicates that the species is growing in its own spent media.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary Figs. 1, 2 and Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Lax, S., Abreu, C.I. & Gore, J. Higher temperatures generically favour slower-growing bacterial species in multispecies communities. Nat Ecol Evol 4, 560–567 (2020). https://doi.org/10.1038/s41559-020-1126-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1126-5
This article is cited by
-
Inter-bacterial mutualism promoted by public goods in a system characterized by deterministic temperature variation
Nature Communications (2023)
-
Stress-induced metabolic exchanges between complementary bacterial types underly a dynamic mechanism of inter-species stress resistance
Nature Communications (2023)
-
Competition contributes to both warm and cool range edges
Nature Communications (2022)
-
Warming and eutrophication interactively drive changes in the methane-oxidizing community of shallow lakes
ISME Communications (2021)
-
Successful microbial colonization of space in a more dispersed manner
ISME Communications (2021)